A 24-week prospective, multicenter, real-world study on eptinezumab's effectiveness and safety in migraine prevention (EMBRACE II)
- PMID: 40332560
- PMCID: PMC12058817
- DOI: 10.1007/s00415-025-13095-z
A 24-week prospective, multicenter, real-world study on eptinezumab's effectiveness and safety in migraine prevention (EMBRACE II)
Abstract
Introduction: We evaluated the effectiveness, tolerability, and safety of eptinezumab in preventing high-frequency episodic migraine (HFEM) and chronic migraine (CM) over 24 weeks in real-world. We also assessed its impact during the first treatment week, in patients failing monoclonal antibodies targeting the calcitonin gene-related peptide (anti-CGRP mAbs), and the effects of dose escalation to 300 mg in patients requiring enhanced control.
Methods: EMBRACE II is a multicenter (n = 22), prospective, 24-week, real-world study involving consecutive patients with HFEM or CM who had failed > 3 preventive treatments. Eptinezumab (100 mg, with the option for escalation to 300 mg at week 12) was administered quarterly.
Primary endpoint: change in monthly migraine days (MMD), for HFEM or monthly headache days (MHD), for CM, between weeks 21-24 and baseline. Secondary endpoints: changes in monthly analgesic intake (MAI), Numerical Rating Scale (NRS), Headache Impact Test (HIT-6), Migraine Disability Assessment Scale (MIDAS), Migraine Interictal Burden Scale (MIBS-4), and responder rates.
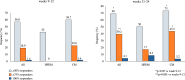
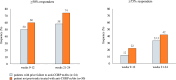
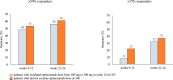
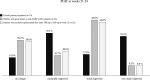
Results: Of the 215 participants who had received ≥ 1 eptinezumab dose, 74 were treated for ≥ 24 weeks and considered for effectiveness analysis. Eptinezumab significantly (p < 0.001) reduced MMD/MHD (- 10.5), MAI (- 15.6), NRS (- 2.2), HIT-6 (- 9.9), MIDAS (- 48.7), and MIBS-4 (- 4.3). ≥ 50% responders were 69%, ≥ 75% responders 39.2%, and 100% responders 4.1%. Comparing the first week with the last baseline week, a significant reduction in migraine days was observed (- 3.7; p < 0.001). Significant improvements were seen in patients failing anti-CGRP mAbs (32.4%) and in those escalating to 300 mg (33.8%). Half of the subjects reported being "very much improved" or "much improved". The adverse events were infrequent (2.8%).
Conclusions: This real-world study documents that 24-week eptinezumab treatment is rapidly effective and well tolerated in migraine patients with multiple therapeutic failures (including anti-CGRP mAbs). One-third of patients escalated to 300 mg at week 12, achieving further significant migraine-related disability reduction.
Keywords: CGRP; Disability; Eptinezumab; Migraine; Real-world; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: Piero Barbanti received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, ElectroCore, Eli-Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, Lusofarmaco, 1MED, MSD, New Penta, Noema Pharma, Novartis, Organon, Orion Pharma, Pfizer, Stx-Med, Teva, Viatris, Visufarma, Zambon and serves as President with Italian Association of Headache Sufferers. Cinzia Aurilia received travel grants from Eli-Lilly, FB-Health, Lusofarmaco and Teva, honoraria from Novartis, Eli-Lilly and Teva. Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta and Ecupharma. Alberto Doretti received travel grants and honoraria from Eli Lilly, Zambon, Teva, Abbvie, Neopharmed Gentili, and Lundbeck. Florindo d’Onofrio received travel grant, honoraria as a speaker or for partecipating in advisory boards from Novartis, Teva, Neopharmed Gentili, Qbgroup srl, K link srl and Eli-Lilly. Mattia Sansone received honorarium for speaker activities from Lundbeck. Giovanna Viticchi received honoraria for speaker activities and participating in advisory boards from Eli-Lilly, AbbVie, Teva and Boehringer Ingelheim. Marco Bartolini received honoraria for speaker activities from Lusofarmaco and Neopharmed. Angelo Ranieri received honoraria for speaker activities, advisory boards, consulting, editorial contribution and travel grants from Novartis, Teva, Eli-Lilly, VyvaMed Srl, CPM Srl, CTP SrlK link srl, Lundbeck, AIM Education Srl, Momento Medico Srl. Davide Mascarella received educational grant from Eli Lilly and travel grant from Abbvie, Eli Lilly, and Teva. Paola Torelli received travel grant, honoraria as a speaker, or for participating in advisory boards from Novartis, Teva, Eli Lilly, and Allergan. Roberta Messina received honoraria for speaker activities and participating in advisory boards from Abbvie, Biomedia, Eli Lilly, Lundbeck, Organon, Pfizer and Teva. Massimo Filippi is the Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almi- rall, Biogen, Horizon, Merck, Novartis, Roche, and Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Horizon, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participa- tion in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Horizon, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Gen- zyme, and Takeda; scientific direction of educational events for Bio- gen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, and Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, Fondazi- one Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Sabina Cevoli received honoraria for speaker panels from Teva and Novartis. Paola Scatena, Steno Rinalduzzi, Luisa Vinciguerra, Rosario Vecchio, Valeria Drago, Monica Laura Bandettini di Poggio, Francesco Baldisseri, Fabio Brusaferri, Luigi Caputi, Stefano Messina, Massimo Autunno, Alessandro Valenza, Bianca Orlando, Marisa Distefano, Laura Borrello, Francesca Pistoia, Cecilia Camarda, Gennaro Saporito, Giacomo Querzola, Antonio Salerno, Francesca Gragnani, Barbara Petolicchio, Antonio Carnevale, Sofia Tavani, Giulia Fiorentini, Stefano Bonassi and Alice Mannocci have no disclosures to declare. Ethical approval: The study was conducted in accordance with principles outlined in the Declaration of Helsinki. The study protocol received approval by Lazio Area 5 Review Board (N. 177/SR/24) and mutually recognized by other local Institutional Review Boards. Informed consent and consent to participate: Eligible participants provided informed consent to participate in the study and were subsequently enrolled. Editorial assistance: The authors would like to thank Liliana Baiamonte and Roberta Borzi for precious support in data management and editorial assistance.
Figures

References
-
- Migraine AM (2020) Migraine. N Engl J Med 383(19):1866–1876 - PubMed
-
- Messina R, Huessler EM, Puledda F, Haghdoost F, Lebedeva ER, Diener HC (2023) Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: a systematic review and network meta-analysis. Cephalalgia 43(3):3331024231152169. 10.1177/03331024231152169 - PubMed
-
- Janković SM, Janković SV (2024) Anti-calcitonin gene-related peptide monoclonal antibodies in migraine: focus on clinical pharmacokinetics. Eur J Drug Metab Pharmacokinet 49(3):277–293. 10.1007/s13318-024-00885-5 - PubMed
-
- Spuntarelli V, Negro A, Luciani M, Bentivegna E, Martelletti P (2021) Eptinezumab for the treatment of migraine. Expert Opin Biol Ther 21(8):999–1011. 10.1080/14712598.2021.1931678 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

