Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower
- PMID: 40332590
- PMCID: PMC12027854
- DOI: 10.3390/ijms26083867
Research Progress of Genomics Applications in Secondary Metabolites of Medicinal Plants: A Case Study in Safflower
Abstract
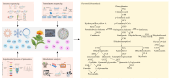
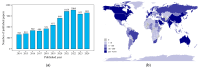
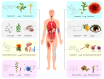
Medicinal plants, recognized as significant natural resources, have gained prominence in response to the increasing global demand for herbal medicines, necessitating the large-scale production of these plants and their derivatives. Medicinal plants are exposed to a variety of internal and external factors that interact to influence the biosynthesis and accumulation of secondary metabolites. With the rapid development of omics technologies such as genomics, transcriptomics, proteomics, and metabolomics, multi-omics technologies have become important tools for revealing the complexity and functionality of organisms. They are conducive to further uncovering the biological activities of secondary metabolites in medicinal plants and clarifying the molecular mechanisms underlying the production of secondary metabolites. Also, artificial intelligence (AI) technology accelerates the comprehensive utilization of high-dimensional datasets and offers transformative potential for multi-omics analysis. However, there is currently no systematic review summarizing the genomic mechanisms of secondary metabolite biosynthesis in medicinal plants. Safflower (Carthamus tinctorius L.) has rich and diverse bioactive flavonoids, among of which Hydroxysafflor yellow A (HSYA) is specific to safflower and emerging as a potential medication for treating a wide range of diseases. Hence, significant progress has been made in the study of safflower as an excellent example for the regulation of secondary metabolites in medicinal plants in recent years. Here, we review the progress on the understanding of the regulation of main secondary metabolites at the multi-omics level, and summarize the influence of various factors on their types and contents, with a particular focus on safflower flavonoids. This review aims to provide a comprehensive insight into the regulatory mechanisms of secondary metabolite biosynthesis from the perspective of genomics.
Keywords: genomics; medicinal plants; regulation; safflower; secondary metabolites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites.Phytomedicine. 2019 May;58:152826. doi: 10.1016/j.phymed.2019.152826. Epub 2019 Jan 9. Phytomedicine. 2019. PMID: 30836217
-
Integrating molecular characterization and metabolites profile revealed CtCHI1's significant role in Carthamus tinctorius L.BMC Plant Biol. 2019 Aug 27;19(1):376. doi: 10.1186/s12870-019-1962-0. BMC Plant Biol. 2019. PMID: 31455221 Free PMC article.
-
The Current Developments in Medicinal Plant Genomics Enabled the Diversification of Secondary Metabolites' Biosynthesis.Int J Mol Sci. 2022 Dec 14;23(24):15932. doi: 10.3390/ijms232415932. Int J Mol Sci. 2022. PMID: 36555572 Free PMC article. Review.
-
Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment.BMC Plant Biol. 2020 Jul 29;20(1):353. doi: 10.1186/s12870-020-02554-6. BMC Plant Biol. 2020. PMID: 32727365 Free PMC article.
-
[Development of Plant Metabolomics and Medicinal Plant Genomics].Yakugaku Zasshi. 2018;138(1):1-18. doi: 10.1248/yakushi.17-00193. Yakugaku Zasshi. 2018. PMID: 29311454 Review. Japanese.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

