Altered protein homeostasis in cardiovascular diseases contributes to Alzheimer's-like neuropathology
- PMID: 40332607
- PMCID: PMC12158837
- DOI: 10.1007/s00395-025-01109-w
Altered protein homeostasis in cardiovascular diseases contributes to Alzheimer's-like neuropathology
Abstract
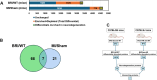
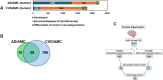
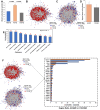
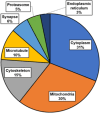
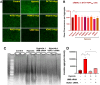
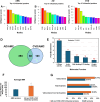
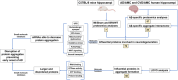
Cardiovascular diseases (CVDs) are the leading cause of death worldwide. CVD is known to increase the risk of subsequent neurodegeneration but the mechanism(s) and proteins involved have yet to be elucidated. We previously showed that myocardial infarction (MI), induced in mice and compared to sham-MI mice, leads to increases in protein aggregation, endoplasmic reticulum (ER) stress in both heart and brain, and changes in proteostatic pathways. In this study, we further investigate the molecular mechanisms altered by induced MI in mice, which were also implicated by proteomics of postmortem human hippocampal aggregates from Alzheimer's disease (AD) and cardiovascular disease (CVD) patients, vs. age-matched controls (AMC). We utilized intra-aggregate crosslinking to identify protein-protein contacts or proximities, and thus to reconstruct aggregate "contactomes" (nonfunctional interactomes). We used leave-one-out analysis (LOOA) to determine the contribution of each protein to overall aggregate cohesion, and gene ontology meta-analyses of constituent proteins to define critical organelles, processes, and pathways that distinguish AD and/or CVD from AMC aggregates. We identified influential proteins in both AD and CVD aggregates, many of which are associated with pathways or processes previously implicated in neurodegeneration such as mitochondrial, oxidative, and endoplasmic-reticulum stress; protein aggregation and proteostasis; the ubiquitin proteasome system and autophagy; axonal transport; and synapses.
Keywords: Alzheimer’s disease; Cardiovascular disease; Crosslinking studies; Leave-one-out analysis; Protein aggregates.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest. Ethical approval: The research presented here was reviewed by the Institutional Review Board of the Central Arkansas Veterans Healthcare System (CAVHS IRB), which deemed it non-human-subject research due to our use of de-identified brain tissue from an approved brain bank, plus mechanistic studies of cultured human cell lines model of AD-like aggregation.
Figures
References
-
- Ayyadevara S, Balasubramaniam M, Parcon PA, Barger SW, Griffin WS, Alla R, Tackett AJ, Mackintosh SG, Petricoin E, Zhou W, Shmookler Reis RJ (2016) Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer’s hippocampus from normal controls. Aging Cell 15:924–939. 10.1111/acel.12501 - DOI - PMC - PubMed
-
- Balasubramaniam M, Ayyadevara S, Ganne A, Kakraba S, Penthala NR, Du X, Crooks PA, Griffin ST, Shmookler Reis RJ (2019) Aggregate interactome based on protein cross-linking interfaces predicts drug targets to limit aggregation in neurodegenerative diseases. iScience 20:248–264. 10.1016/j.isci.2019.09.026 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

