Amylin: From Mode of Action to Future Clinical Potential in Diabetes and Obesity
- PMID: 40332747
- PMCID: PMC12085449
- DOI: 10.1007/s13300-025-01733-8
Amylin: From Mode of Action to Future Clinical Potential in Diabetes and Obesity
Abstract
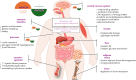
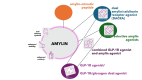
Precision diabetology is increasingly becoming diabetes phenotype-driven, whereby the specific hormonal imbalances involved are taken into consideration. Concomitantly, body weight-favorable therapeutic approaches are being dictated by the obesity pandemic, which extends to all diabetes subpopulations. Amylin, an anorexic neuroendocrine hormone co-secreted with insulin, is deficient in individuals with diabetes and plays an important role in postprandial glucose homeostasis, with additional potential cardiovascular and neuroprotective functions. Its actions include suppressing glucagon secretion, delaying gastric emptying, increasing energy expenditure and promoting satiety. While amylin holds promise as a therapeutic agent, its translation into clinical practice is hampered by complex receptor biology, the limitations of animal models, its amyloidogenic properties and pharmacokinetic challenges. In individuals with advanced β-cell dysfunction, supplementing insulin therapy with pramlintide, the first and currently only approved injectable short-acting selective analog of amylin, has demonstrated efficacy in enhancing both postprandial and overall glycemic control in both type 2 diabetes (T2D) and type 1 diabetes (T1D) without increasing the risk of hypoglycemia or weight gain. Current research focuses on several key strategies, from enhancing amylin stability by attaching polyethylene glycol or carbohydrate molecules to amylin, to developing oral amylin formulations to improve patients' convenience, as well as developing various combination therapies to enhance weight loss and glucose regulation by targeting multiple receptors in metabolic pathways. The novel synergistically acting glucagon-like peptide-1 (GLP-1) receptor agonist combined with the amylin agonist, CagriSema, shows promising results in both glucose regulation and weight management. As such, amylin agonists (combined with other members of the incretin class) could represent the elusive drug candidate to address the multi-hormonal dysregulations of diabetes subtypes and qualify as a precision medicine approach that surpasses the long overdue division into T1DM and T2DM. Further development of amylin-based therapies or delivery systems is crucial to fully unlock the therapeutic potential of this intriguing hormone.Graphical abstract available for this article.
Keywords: Adjunct non-insulin-based therapies; Amylin; Amylin receptor agonists; Cagrisema; Incretin; Obesity; Postprandial hyperglycemia; Pramlintide; Type 1 diabetes mellitus; Type 2 diabetes mellitus.
Plain language summary
Diabetes is a chronic disease with many faces, and persons affected by diabetes are continuously confronted with challenges, with injecting insulin and fighting obesity being the most recent. Current research is focused on designing drug candidates that would simultaneously address different mechanisms of high glucose in an individual and contribute to satiation, body weight control and patient convenience. Amylin is a hormone that is, similar to insulin, secreted by the pancreas. Its clinical use is currently limited to a single product, known as pramlintide, which is approved as an adjunct therapy in persons with insulin-treated diabetes. While the use of amylin is efficient, patients have to inject it with each meal and in a separate injection from insulin. Consequently, improving amylin-based medicines focuses on enabling longer action, resulting in weekly use, possibly even oral use and, most importantly, combining the functions of more than one hormone known for being involved in glucose control and the promotion of satiety. Similarly to other medicines primarily used in persons with diabetes, amylin-based medicines might eventually be used to address different diseases of the metabolic spectrum, namely obesity and its complications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: All authors have given lectures, received honoraria and research support and participated in conferences, advisory boards and clinical trials sponsored by many pharmaceutical companies. However, no pharmaceutical company played any role in the scientific content of the present article, which has been written independently and reflects only the opinions of the authors, without no imput from the pharmaceutical industry. The following conflict of interests are reported. Špela Volčanšek has received lecture honoraria from Novo Nordisk, Eli Lilly, Medtronic, Abbott, AstraZeneca and Boehringer Ingelheim. Andrijana Koceva reports receiving lecture honoraria from Novo Nordisk, Eli Lilly, AstraZeneca, Boehringer Ingelheim, Medtronic and Pfizer. Mojca Jensterle has received lecture honoraria from Novo Nordisk, Eli Lilly, Pfizer, Amgen, Novartis and Sanofi, and is an advisory board member of Novo Nordisk, Eli Lilly, Amgen and Pfizer. Andrej Janež has served as a consultant and is on Speakers Bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic, Abbott, Novo Nordisk, Novartis and Swixx. Emir Muzurović has given lectures and participated in conferences and advisory boards sponsored by several pharmaceutical companies, including Novo Nordisk, Sanofi, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Medtronic and Servier. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures


Similar articles
-
Inhibition of glucagon secretion.Adv Pharmacol. 2005;52:151-71. doi: 10.1016/S1054-3589(05)52008-8. Adv Pharmacol. 2005. PMID: 16492545 Review.
-
Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control.Curr Pharm Des. 2001 Sep;7(14):1353-73. doi: 10.2174/1381612013397357. Curr Pharm Des. 2001. PMID: 11472273 Review.
-
Pramlintide: profile of an amylin analog.Expert Rev Endocrinol Metab. 2012 Nov;7(6):599-609. doi: 10.1586/eem.12.50. Expert Rev Endocrinol Metab. 2012. PMID: 30754127
-
Role of Amylin in Type 1 and Type 2 Diabetes.Diabetes Educ. 2015 Dec;41(1 Suppl):47S-56S. doi: 10.1177/0145721715607642. Epub 2015 Sep 30. Diabetes Educ. 2015. PMID: 26424675 Review.
-
Pramlintide acetate in the treatment of Type 2 and Type 1 diabetes mellitus.Expert Rev Endocrinol Metab. 2007 Jan;2(1):9-18. doi: 10.1586/17446651.2.1.9. Expert Rev Endocrinol Metab. 2007. PMID: 30743744
References
-
- Leslie RD, Ma RCW, Franks PW, Nadeau KJ, Pearson ER, Redondo MJ. Understanding diabetes heterogeneity: key steps towards precision medicine in diabetes. Lancet Diabetes Endocrinol. 2023;11:848–60. https://www.thelancet.com/journals/landia/article/PIIS2213-8587(23)00159.... - PubMed
-
- Stidsen JV, Henriksen JE, Olsen MH.., et al. Pathophysiology-based phenotyping in type 2 diabetes: a clinical classification tool. Diabetes Metab Res Rev. 2018;34:e3005. - PubMed
-
- Drucker DJ, Holst JJ. The expanding incretin universe: from basic biology to clinical translation. Diabetologia. 2023;66:1765–79. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

