Roflumilast Foam, 0.3%, for Psoriasis of the Scalp and Body: The ARRECTOR Phase 3 Randomized Clinical Trial
- PMID: 40332898
- PMCID: PMC12060019
- DOI: 10.1001/jamadermatol.2025.1136
Roflumilast Foam, 0.3%, for Psoriasis of the Scalp and Body: The ARRECTOR Phase 3 Randomized Clinical Trial
Abstract
Importance: Current topical treatments for scalp psoriasis are limited by formulation, efficacy, and/or safety.
Objective: To assess safety and efficacy of roflumilast foam, 0.3%, in patients with psoriasis of the scalp and body.
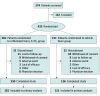
Design, setting, and participants: This was a phase 3 double-blinded, vehicle-controlled randomized clinical trial conducted between August 24, 2021, and June 3, 2022, at 49 sites in Canada and the US. Eligible participants were 12 years and older with plaque psoriasis affecting up to 25% of the scalp and body, at least 10% of the scalp, and up to 20% of nonscalp areas, with a minimum Scalp-Investigator Global Assessment (S-IGA) score of 3 (moderate), and minimum Body-IGA (B-IGA) score of 2 (mild). Data analyses were performed from September 9 to December 30, 2022.
Interventions: Once-daily roflumilast foam, 0.3%, or vehicle for 8 weeks.
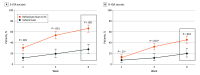
Main outcomes and measures: Coprimary end points were S-IGA and B-IGA success (clear [0] or almost clear [1] plus ≥2-grade improvement) at week 8. Secondary end points included S-IGA success at weeks 2 and 4, change in Scalp Itch-Numeric Rating Scale (SI-NRS), and SI-NRS and Worst Itch-NRS (WI-NRS) success (≥4-point improvement in patients with baseline score of ≥4). Safety and tolerability were also assessed.
Results: A total of 432 patients (mean [SD] age, 47.3 [14.8] years; 243 women [56.3%]) were randomized to roflumilast foam (n = 281) or vehicle (n = 151). At week 8, 66.4% of the roflumilast group achieved S-IGA success vs 27.8% of the vehicle group (P < .001); and 45.5% of the roflumilast group achieved B-IGA success compared with 20.1% of the vehicle group (P < .001). Rates for S-IGA success at week 2 and SI-NRS and WI-NRS success at weeks 2, 4, and 8 were significantly higher for roflumilast vs vehicle. Improvements in SI-NRS were greater for the roflumilast vs the vehicle group as early as the first assessment (24 hours after the first application). Both study groups had low rates of adverse events and favorable tolerability profiles.
Conclusions and relevance: This randomized clinical trial found that roflumilast foam, 0.3%, improved signs and symptoms of psoriasis on the scalp and body, including pruritus, with low rates of adverse events in patients 12 years and older. These results demonstrate the potential of roflumilast foam, 0.3%, as monotherapy for patients with psoriasis of the scalp and body.
Trial registration: ClinicalTrials.gov Identifier: NCT05028582.
Conflict of interest statement
Figures

References
-
- Armstrong A, Young M, Seal MS, Higham RC, Greiling T. Treatment burden and the perspectives of patients with psoriasis using topical treatments: results from a national survey of adults with psoriasis in the United States. J Dermatolog Treat. 2024;35(1):2389174. doi: 10.1080/09546634.2024.2389174 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

