Glucagon-Like Peptide 1 Receptor Agonist Stimulation Inhibits Laser-Induced Choroidal Neovascularization by Suppressing Intraocular Inflammation
- PMID: 40332908
- PMCID: PMC12061060
- DOI: 10.1167/iovs.66.5.15
Glucagon-Like Peptide 1 Receptor Agonist Stimulation Inhibits Laser-Induced Choroidal Neovascularization by Suppressing Intraocular Inflammation
Abstract
Purpose: The glucagon-like peptide-1 receptor (GLP-1R), a diabetes therapy target, is expressed in multiple organs and is associated with neuroprotective, anti-inflammatory, and antitumor effects, particularly in cardiac and cerebral tissues. Although GLP-1's role in diabetic and ischemic retinopathies is well-studied, its influence on choroidal neovascularization (CNV) in exudative age-related macular degeneration (AMD) remains unclear. This study explored the effects of GLP-1 on CNV using a laser-induced mouse model.
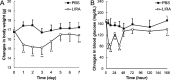
Methods: The anti-angiogenic effects of GLP-1 were tested using ex vivo sprouting assays in 3-week-old C57BL/6J mice. In 6-week-old mice, GLP-1R localization in laser-induced CNV lesions was analyzed via immunohistochemistry. Liraglutide, a GLP-1R agonist, was administered subcutaneously for 7 days or by single intravitreal injection post-laser. Eyeballs collected on days 1 to 7 post-laser were analyzed using RT-qPCR for GLP-1R expression and inflammatory cytokines.
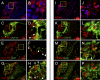
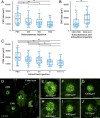
Results: GLP-1R-positive cells were detected in CNV lesions and were expressed in Iba-1-positive activated microglia or macrophages. They also expressed in abnormal retinal pigment epithelial cells and surrounding normal endothelial cells. NOD-like receptor protein 3 (NLRP3) inflammasome signaling was observed near CNV. Liraglutide inhibited angiogenesis in ex vivo assays and significantly reduced CNV formation with both subcutaneous and intravitreal administration. Additionally, Liraglutide inhibited expression of NLRP3, IL-1β, IL-6, and TNF expression compared with healthy controls. Intravitreal GLP-1R antagonist reduced subcutaneous effects.
Conclusions: Liraglutide suppresses CNV formation, likely via NLRP3 inflammasome inhibition. Intraocular GLP-1R appears to mediate anti-CNV effects, supporting GLP-1R agonists as potential adjunctive therapy for exudative AMD and warranting further investigation into its safety and clinical feasibility.
Conflict of interest statement
Disclosure:
Figures



References
-
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004; 291: 1900–1901. - PubMed
-
- de Jong PT. Age-related macular degeneration. N Engl J Med. 2006; 355: 1474–1485. - PubMed
-
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY.. Age-related macular degeneration. Lancet. 2012; 379: 1728–1738. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. .. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1432–1444. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. .. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355: 1419–1431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

