Adipokinetic Hormones and Their Receptor Regulate the Locomotor Behavior in Tribolium castaneum
- PMID: 40332910
- PMCID: PMC12028090
- DOI: 10.3390/insects16040407
Adipokinetic Hormones and Their Receptor Regulate the Locomotor Behavior in Tribolium castaneum
Abstract
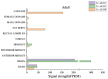
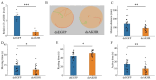
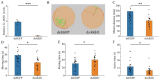
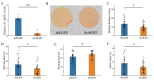
The regulation of locomotor behavior is essential for insects to perform their life activities. The central nervous system plays a pivotal role in modulating physiological behaviors, particularly movement, with neuropeptides serving as key modulators of these processes. Among these, adipokinetic hormone (AKH) was originally identified in insects as a neurohormone involved in lipid mobilization. This study investigates the functional role of AKHs (AKH1 and AKH2) and their receptor (AKHR) in regulating locomotion in the red flour beetle, Tribolium castaneum. Using functional calcium reporter assays, we demonstrated that AKHR is activated by two mature AKH peptides from T. castaneum, with half-maximal effective concentrations (EC50) falling within the nanomolar range. Gene expression analysis confirmed the presence of AKH1 and AKH2 transcripts in the brain, while AKHR expression was localized to the fat body and carcass. The silencing of AKHs or AKHR through RNA interference resulted in a significant reduction in both movement distance and duration. Collectively, these findings highlight the regulatory influence of AKH/AKHR signaling in locomotor activity in T. castaneum, thereby advancing our understanding of the molecular mechanisms underlying locomotor control in this economically important insect species.
Keywords: RNAi; locomotor behavior; neuropeptide; red flour beetle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

