Centrifugation-Based Purification Protocol Optimization Enhances Structural Preservation of Nucleopolyhedrovirus Budded Virion Envelopes
- PMID: 40332984
- PMCID: PMC12027964
- DOI: 10.3390/insects16040424
Centrifugation-Based Purification Protocol Optimization Enhances Structural Preservation of Nucleopolyhedrovirus Budded Virion Envelopes
Abstract
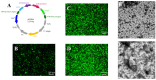
The structural integrity of viral envelopes is a critical determinant of infectivity for enveloped viruses, directly influencing vector stability, functional accuracy of surface-displayed epitopes, and preservation of native conformational states required for membrane protein studies. However, conventional purification methods often disrupt envelope integrity and cause envelope proteins to lose their activity. Here, we systematically compared discontinuous, continuous, and optimized continuous sucrose density gradient centrifugation protocols for purifying Autographa californica multiple nucleopolyhedrovirus (AcMNPV). Through cryo-EM, we demonstrated that our optimized continuous sucrose gradient protocol significantly increased the proportion of AcMNPV budded virions with intact envelopes from 36% to 81%, while preserving the metastable prefusion conformation of the fusion protein GP64. This advancement should prove useful for structural studies of viral envelope proteins and may enhance applications in gene therapy and vaccine development utilizing enveloped viruses.
Keywords: AcMNPV; Alphabaculovirus; baculovirus purification; cryo-EM; sucrose density gradient; viral envelope integrity; virion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Knipe D.M., Howley P.M. Fields Virology. 6th ed. Wolters Kluwer Health—Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013.
Grants and funding
LinkOut - more resources
Full Text Sources

