Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection
- PMID: 40333073
- PMCID: PMC12030701
- DOI: 10.3390/pathogens14040312
Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection
Abstract
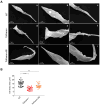
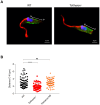
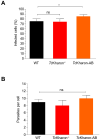
Chagas disease, caused by Trypanosoma cruzi, is a neglected tropical disease with few options for treatment and no available vaccine. Deletion mutants for live attenuated vaccines, particularly deletions of proteins related to the cytoskeleton, have been widely tested in related parasites but candidates have not been tested in T. cruzi. Kharon is one such protein, identified as being associated with the cytoskeleton in Leishmania and essential for amastigote replication. Here we investigated the T. cruzi Kharon ortholog (TcKharon) to test if it has orthologous function and thus potential in generating a live attenuated vaccine. In silico analysis predicted TcKharon to be an intrinsically disordered protein, consistent with its ortholog feature, and GFP fusion protein revealed that TcKharon is associated with the cytoskeleton of epimastigotes. CRISPR-Cas9-mediated gene disruption impaired epimastigote proliferation and cytokinesis, resulting in altered nucleus-to-kinetoplast ratios and pronounced morphological defects, particularly in the posterior cell region. Despite these abnormalities, TcKharon-/- mutants retained the ability to differentiate into metacyclic trypomastigotes and exhibited in vitro infection rates comparable to wild-type parasites. Our data show that TcKharon is crucial for cell morphology. However, in contrast to close related parasites, TcKharon is not essential for in vitro infectivity.
Keywords: CRISPR/Cas9; Trypanosoma cruzi; morphology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Rios L., Campos E.E., Menon R., Zago M.P., Garg N.J. Epidemiology and pathogenesis of maternal-fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta Mol. Basis. Dis. 2020;1866:165591. doi: 10.1016/j.bbadis.2019.165591. - DOI - PMC - PubMed
-
- Junqueira C., Santos L.I., Galvão-Filho B., Teixeira S.M., Rodrigues F.G., DaRocha W.D., Chiari E., Jungbluth A.A., Ritter G., Gnjatic S., et al. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc. Natl. Acad. Sci. USA. 2011;108:19695–19700. doi: 10.1073/pnas.1110030108. - DOI - PMC - PubMed
-
- Almeida A.P.M.M., Machado L.F.M., Doro D., Nascimento F.C., Damasceno L., Gazzinelli R.T., Fernandes A.P., Junqueira C. New vaccine formulations containing a modified version of the Amastigote 2 antigen and the non-virulent Trypanosoma cruzi CL-14 strain are highly antigenic and protective against Leishmania infantum challenge. Front. Immunol. 2018;9:465. doi: 10.3389/fimmu.2018.00465. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

