Essential role of hepcidin in host resistance to disseminated candidiasis
- PMID: 40333187
- PMCID: PMC12172023
- DOI: 10.1016/j.celrep.2025.115649
Essential role of hepcidin in host resistance to disseminated candidiasis
Abstract
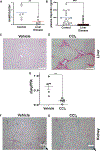
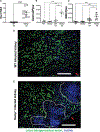
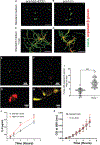
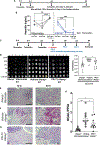
Candida albicans is a leading cause of life-threatening invasive infection despite antifungal therapy. Patients with chronic liver disease are at increased risk of candidemia, but the mechanisms underlying this susceptibility are incompletely defined. One consequence of chronic liver disease is an attenuated ability to produce hepcidin and maintain organismal control of iron homeostasis. To address the biology underlying this critical clinical problem, we demonstrate the mechanistic link between hepcidin insufficiency and candida infection using genetic and inducible hepcidin knockout mice. Hepcidin deficiency led to unrestrained fungal growth and increased transition to the invasive hypha morphology with exposed 1,3-β-glucan, which exacerbated kidney injury, independent of the fungal pore-forming toxin candidalysin in immunocompetent mice. Of translational relevance, the therapeutic administration of PR-73, a hepcidin mimetic, improved the outcome of infection. Thus, we identify hepcidin deficiency as a host susceptibility factor against C. albicans and hepcidin mimetics as a potential intervention.
Keywords: 1,3; CP: Metabolism; CP: Microbiology; Candida albicans; PR73; chronic liver disease; hepcidin; hepcidin mimetic; iron; β-glucan.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Y.S. is a consultant for Disc Medicine, and E.N. is a scientific co-founder of Intrinsic LifeSciences and Silarus Therapeutics and a consultant for Vifor, Protagonist, Ionis, Disc Medicine, GSK, Novo Nordisk, Chiesi, and Dogodan Therapeutics.
Figures



Update of
-
Essential role of Hepcidin in host resistance to disseminated candidiasis.bioRxiv [Preprint]. 2024 Oct 29:2024.10.29.620511. doi: 10.1101/2024.10.29.620511. bioRxiv. 2024. Update in: Cell Rep. 2025 May 27;44(5):115649. doi: 10.1016/j.celrep.2025.115649. PMID: 39553949 Free PMC article. Updated. Preprint.
References
-
- Delavy M, Sertour N, Patin E, Le Chatelier E, Cole N, Dubois F, Xie Z, Saint-André V, Manichanh C, Walker AW, et al. (2023). Unveiling Candida albicans intestinal carriage in healthy volunteers: the role of micro- and mycobiota, diet, host genetics and immune response. Gut Microbes 15, 2287618. 10.1080/19490976.2023.2287618. - DOI - PMC - PubMed
-
- Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, Wenzel RP, and Seifert H (2014). Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 43, 78–81. 10.1016/j.ijantimicag.2013.09.005. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

