High Rate of Antibody Response to Multiple Doses of the COVID-19 Vaccine in Liver Transplant Recipients: Analysis of Predictive Factors
- PMID: 40333209
- PMCID: PMC12030985
- DOI: 10.3390/vaccines13040352
High Rate of Antibody Response to Multiple Doses of the COVID-19 Vaccine in Liver Transplant Recipients: Analysis of Predictive Factors
Abstract
Background: The COVID-19 pandemic highlighted the vulnerability of immunocompromised individuals, including liver transplant recipients (LTRs), who often exhibit reduced vaccine immunogenicity. While initial vaccine doses and subsequent boosters improved immune response, LTRs were prioritized for vaccination. Studies have shown increased antibody response after each booster dose. Vaccine hesitancy, defined as delayed or refused vaccination despite availability, poses a public health challenge, often fueled by misinformation. This study aimed to evaluate anti-spike antibody responses in vaccinated LTRs after two initial doses and at least one booster, also assessing adherence to subsequent doses.
Methods: We conducted a retrospective observational study at a transplant center in Milan, Italy, between January 2021 and December 2023. LTRs who had received four or more doses of mRNA vaccines (Pfizer or Moderna) were included. Anti-spike antibody levels were measured 60-80 days after each dose. Data on vaccination status were collected in January 2024. Statistical analysis was performed to compare antibody responses and identify predictive factors.
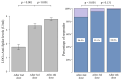
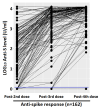
Results: LTRs showed a significant increase in anti-spike antibody responses after the first booster compared to the second dose with a trend versus a further increase following the fourth dose in a subgroup of the patients receiving two booster doses. However, adherence to booster doses decreased over time. In LTRs, predictors of a weaker response after the second dose were chronic kidney disease and metabolic etiology at transplant.
Conclusions: The study highlighted that in LTRs, multiple doses of the COVID-19 vaccine led to a continuous increase in anti-spike antibody responses. The progressive decline in adherence of LTRs "to further booster doses" should be related to the fact that after the spread of vaccination programs worldwide, COVID-19 is still a current infection, but it is much less severe than before.
Keywords: COVID-19; first booster; fourth dose; liver transplant recipients; predictive factors; second booster; vaccination; vaccine hesitancy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- San Francisco Ramos A., Liu Sanchez C., Bovill Rose T., Smith D., Thorn N., Galiza E., Miah T., Pearce J., Hultin C., Cosgrove C., et al. Comparing reactogenicity of COVID-19 vaccine boosters: A systematic review and meta-analysis. Expert Rev. Vaccines. 2024;23:266–282. doi: 10.1080/14760584.2024.2315089. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

