An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus
- PMID: 40333228
- PMCID: PMC12031407
- DOI: 10.3390/vaccines13040374
An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus
Abstract
Background: Since the outbreak of mpox in 2022, the disease has spread rapidly worldwide and garnered significant public attention. Vaccination is regarded as an effective measure to prevent the spread of mpox. The success of the COVID-19 mRNA vaccine demonstrates that mRNA-based vaccines represent a rapid and multifunctional platform with considerable potential, and are expected to be a strategy to address mpox spread.
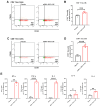
Methods: In this study, we screened an mpox multi-antigen-tandem bivalent mRNA vaccine candidate: a lipid nanoparticle-encapsulated mRNA-1017 and mRNA-1995 (mRNA-3012-LNP). We then evaluated the immunogenicity of the mpox virus (MPXV) bivalent mRNA vaccine candidate and its protective efficacy against the vaccinia virus (VACV) in a mouse model.
Results: Mice vaccinated with two doses of the mRNA-3012-LNP vaccine exhibited robust binding antibody responses and MPXV-specific Th-1-biased cellular immune responses in vivo. Notably, the boosted immunized mice generated potent neutralizing antibodies against the VACV, effectively protecting them from viral challenge. Additionally, serum transfer protection experiments indicated that serum from mice inoculated with mRNA-3012-LNP was effective in protecting nude mice from VACV challenge.
Conclusions: Our results suggest that the mpox bivalent mRNA candidate vaccine mRNA-3012-LNP induces strong immunogenicity and has the potential to serve as a safe and effective vaccine candidate against mpox epidemics.
Keywords: VACV; bivalent; mRNA vaccine; mpox; tandem.
Conflict of interest statement
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Figures




Similar articles
-
Long-Lasting Protection and Dose Optimization of MPXV Polyvalent Mpox mRNA Vaccines Against Lethal Vaccinia Virus Challenge in Mice.J Med Virol. 2025 Jan;97(1):e70143. doi: 10.1002/jmv.70143. J Med Virol. 2025. PMID: 39726255
-
Polyvalent mpox mRNA vaccines elicit robust immune responses and confer potent protection against vaccinia virus.Cell Rep. 2024 Jun 25;43(6):114269. doi: 10.1016/j.celrep.2024.114269. Epub 2024 May 23. Cell Rep. 2024. PMID: 38787725
-
A Single-Chain Mpox mRNA Vaccine Elicits Protective Immune Response in Mice.Vaccines (Basel). 2025 May 13;13(5):514. doi: 10.3390/vaccines13050514. Vaccines (Basel). 2025. PMID: 40432124 Free PMC article.
-
Current Status of Vaccine Development for Monkeypox Virus.Adv Exp Med Biol. 2024;1451:289-300. doi: 10.1007/978-3-031-57165-7_18. Adv Exp Med Biol. 2024. PMID: 38801585 Review.
-
An overview on mRNA-based vaccines to prevent monkeypox infection.J Nanobiotechnology. 2024 Mar 1;22(1):86. doi: 10.1186/s12951-024-02355-1. J Nanobiotechnology. 2024. Retraction in: J Nanobiotechnology. 2024 Jul 9;22(1):402. doi: 10.1186/s12951-024-02693-0. PMID: 38429829 Free PMC article. Retracted. Review.
References
-
- Second Meeting of the International Health Regulations (2005) (IHR) Emergency Committee Regarding the Multi-Country Outbreak of Monkeypox. [(accessed on 19 August 2024)]. Available online: https://www.who.int/news/item/23-07-2022-second-meeting-of-the-internati....
-
- Fifth Meeting of the International Health Regulations (2005) (IHR) Emergency Committee on the Multi-Country Outbreak of mpox (Monkeypox) [(accessed on 6 November 2024)]. Available online: https://www.who.int/news/item/11-05-2023-fifth-meeting-of-the-internatio...
-
- Kibungu E.M., Vakaniaki E.H., Kinganda-Lusamaki E., Kalonji-Mukendi T., Pukuta E., Hoff N.A., Bogoch I.I., Cevik M., Gonsalves G.S., Hensley L.E., et al. Clade I–Associated Mpox Cases Associated with Sexual Contact, the Democratic Republic of the Congo. Emerg. Infect. Dis. 2024;30:172–176. doi: 10.3201/eid3001.231164. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

