The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis
- PMID: 40333247
- PMCID: PMC12031506
- DOI: 10.3390/vaccines13040368
The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis
Abstract
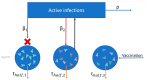
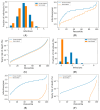
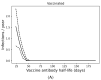
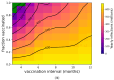
Background: While the rapid deployment of SARS-CoV-2 vaccines had a significant impact on the ongoing COVID-19 pandemic, rapid viral immune evasion and waning neutralizing antibody titers have degraded vaccine efficacy. Nevertheless, vaccine manufacturers and public health authorities have a number of options at their disposal to maximize the benefits of vaccination. In particular, the effect of booster schedules on vaccine performance bears further study. Methods: To better understand the effect of booster schedules on vaccine performance, we used an agent-based modeling framework and a population pharmacokinetic model to simulate the impact of boosting frequency on the durability of vaccine protection against infection and severe acute disease. Results: Our work suggests that repeated dosing at frequent intervals (three or more times a year) may offset the degradation of vaccine efficacy, preserving the utility of vaccines in managing the ongoing pandemic. Conclusions: Given the practical significance of potential improvements in vaccine utility, clinical research to better understand the effects of repeated vaccination would be highly impactful. These findings are particularly relevant as public health authorities worldwide have reduced the frequency of boosters to once a year or less.
Keywords: COVID-19; SARS-CoV-2; agent-based modeling; scheduling; vaccination; vaccine boosting.
Conflict of interest statement
M.S., L.Y., and A.C. are employees of and shareholders in Fractal Therapeutics; R.P.N. is a shareholder of Fractal Therapeutics and an employee of Halozyme Therapeutics. G.H. is an employee of Sage Therapeutics. Neither Fractal Therapeutics, Halozyme Therapeutics, nor Sage Therapeutics has any business interest in the subject of this paper.
Figures




Similar articles
-
Diversified humoral immunity and impacts of booster vaccines: SARS-CoV-2 antibody profile and Omicron BA.2 neutralization before and after first or second boosters.Microbiol Spectr. 2024 Oct 3;12(10):e0060524. doi: 10.1128/spectrum.00605-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162540 Free PMC article.
-
Impact of the COVID-19 vaccine booster strategy on vaccine protection: a pilot study of a military hospital in Taiwan.Clin Exp Vaccine Res. 2023 Oct;12(4):337-345. doi: 10.7774/cevr.2023.12.4.337. Epub 2023 Oct 31. Clin Exp Vaccine Res. 2023. PMID: 38025918 Free PMC article.
-
Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis.Lancet Microbe. 2022 Jan;3(1):e52-e61. doi: 10.1016/S2666-5247(21)00267-6. Epub 2021 Nov 15. Lancet Microbe. 2022. PMID: 34806056 Free PMC article.
-
Real-world evidence on the efficacy of bivalent booster doses of SARS-CoV-2 vaccine in respect of monovalent boosters or primary cycle of vaccination: a narrative review.Epidemiol Prev. 2023 Nov-Dec;47(6):331-343. doi: 10.19191/EP23.6.A626.081. Epidemiol Prev. 2023. PMID: 38314543 Review. English.
-
A Complementary Union of SARS-CoV2 Natural and Vaccine Induced Immune Responses.Front Immunol. 2022 Jul 13;13:914167. doi: 10.3389/fimmu.2022.914167. eCollection 2022. Front Immunol. 2022. PMID: 35911696 Free PMC article. Review.
Cited by
-
COVID-19 Vaccine Timing and Co-Administration with Influenza Vaccines in Canada: A Systematic Review with Comparative Insights from G7 Countries.Vaccines (Basel). 2025 Jun 21;13(7):670. doi: 10.3390/vaccines13070670. Vaccines (Basel). 2025. PMID: 40733647 Free PMC article. Review.
References
-
- Tenforde M.W., Olson S.M., Self W.H., Talbot H.K., Lindsell C.J., Steingrub J.S., Shapiro N.I., Ginde A.A., Douin D.J., Prekker M.E., et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. - DOI - PMC - PubMed
-
- Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. [(accessed on 18 September 2023)]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-an....
LinkOut - more resources
Full Text Sources
Miscellaneous

