Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial
- PMID: 40333248
- PMCID: PMC12031432
- DOI: 10.3390/vaccines13040390
Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial
Abstract
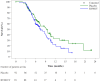
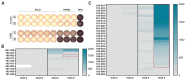
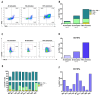
Background: A previous phase I/II study demonstrated potent and long-term immune responses in men with prostate cancer following vaccination with a 20mer synthetic peptide (RV001) derived from the Ras homolog gene family member C protein (RhoC). Moreover, a fraction of patients experienced prostate-specific antigen (PSA) responses, which prompted the initiation of a phase II double-blind randomized trial (NCT04114825). The primary endpoint was to study whether vaccination could postpone PSA progression. Furthermore, the study included an evaluation of vaccination-induced immune responses, and in-depth in vitro studies of RhoC-specific CD4+ T cell responses. Methods: Men with non-metastatic biochemical recurrence after either radical prostatectomy or radiation therapy were eligible for the study. Participants were randomized 1:1 to either subcutaneous injections of 0.1 mg/mL RV001 emulsified in Montanide ISA 51, or a placebo. Vaccinations were administered every 2 weeks for the first six times, then five times every 4 weeks for a total treatment time of 30 weeks. Blood samples were collected from a subset of patients (n = 38) over the course of vaccination, and peripheral blood mononuclear cells (PBMCs) isolated for immunological assessment of vaccine-induced immune responses. Experiments using PBMCs from a healthy donor and a patient were performed to study the phenotype and function of RV001-specific CD4+ T cells. Results: A total of 192 men entered the study. There was no difference in time to PSA doubling, with 7.5 versus 9.3 months, or in time to initiating further therapies, 11.2 versus 17.6 months for treatment and control groups, respectively. At long-term follow-up, 12.9% of the patients in the vaccination arm had developed metastasis compared to 12% in the placebo arm. No serious treatment-related side effects were observed, and treatment-related adverse events did not differ between groups. Immunological examinations in a subset of patients demonstrated that the vaccination induced potent, long-lasting CD4+ T cell responses capable of proliferation and cytokine production. RV001-specific CD4+ T cells were shown to mediate cytotoxicity against a RhoC-expressing cancer cell line in an HLA-class II-dependent manner. Conclusions: Men randomized to active treatment with RV001V demonstrated the induction of potent, functionally capable, anti RhoC-CD4+ T cell responses. However, there was no benefit in time to biochemical progression, and no difference in time to the initiation of second-line therapies.
Keywords: CD4+ T cells; RhoC; cancer vaccine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Noguchi M., Fujimoto K., Arai G., Hashine K., Matsumoto H., Fukasawa S., Kohjimoto Y., Nakatsu H., Takenaka A., Fujisawa M., et al. A randomized phase III trial of personalized peptide vaccination for castration-resistant prostate cancer progressing after docetaxel. Oncol. Rep. 2020;45:159–168. doi: 10.3892/or.2020.7847. - DOI - PMC - PubMed
-
- Wood L.V., Fojo A., Roberson B.D., Hughes M.S.B., Dahut W., Gulley J.L., Madan R.A., Arlen P.M., Sabatino M., Stroncek D.F., et al. TARP vaccination is associated with slowing in PSA velocity and decreasing tumor growth rates in patients with Stage D0 prostate cancer. OncoImmunology. 2016;5:e1197459. doi: 10.1080/2162402X.2016.1197459. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

