Humanized Major Histocompatibility Complex Transgenic Mouse Model Can Play a Potent Role in SARS-CoV-2 Human Leukocyte Antigen-Restricted T Cell Epitope Screening
- PMID: 40333292
- PMCID: PMC12031200
- DOI: 10.3390/vaccines13040416
Humanized Major Histocompatibility Complex Transgenic Mouse Model Can Play a Potent Role in SARS-CoV-2 Human Leukocyte Antigen-Restricted T Cell Epitope Screening
Abstract
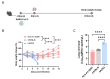
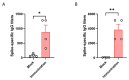
Background: COVID-19, caused by SARS-CoV-2, poses a significant threat to human health. Vaccines designed for T-cell epitopes play an important role in eliminating the virus. However, T cell epitope screening often requires the use of a large number of peripheral blood mononuclear cells (PBMCs) from infected or convalescent patients, and if MHC humanized mice can be used for epitope screening, they will not have to wait for enough PBMCs to be available to screen for epitopes, thus buying time for epitope confirmation and vaccine design. Methods: In this study, we used SARS-CoV-2 BA.5 to infect HLA-A11/DR1, C57BL/6, hACE2 mice, and detected body weight changes, viral load, and pathological changes after infection. Fourteen days after the HLA-A11/DR1 and C57BL/6 mice were immunized against inactivated viruses, IgG antibodies were detected in mouse serum using ELISA, and IFN-γ produced by peptide stimulation of splenocytes was detected by ELISpot. Results: There is no obvious pathogenic phenotype of SARS-CoV-2 infection in HLA-A11/DR1 mice. Specific IgG antibodies were detected in serum after immunization of inactivated virus in both HLA-A11/DR1 and C57BL/6 mice, but specific IFN-γ was detected in splenocytes of HLA-A11/DR1 mice. Conclusions: Although HLA-A11/DR1 mice are unable to replicate the virus effectively in vivo, they are able to generate cellular immune responses after immunization inactivated viruses. Therefore, it can be used as a tool to substitute for human PBMCs in epitope screening, thus shortening the timeliness of T cell epitope screening and obtaining the immunogenicity information of new epitopes in a timely manner.
Keywords: SARS-CoV-2; epitope; human leukocyte antigen (HLA) complex; immunological evaluation; mouse model.
Conflict of interest statement
Hanxiong Qin and Dongmei Zhao are employed by Changchun Institute of Biological Products Co., Ltd. The remaining authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Application of Humanized MHC Transgenic Mice in the Screening of HLA-Restricted T Cell Epitopes for Influenza Vaccines.Vaccines (Basel). 2025 Mar 20;13(3):331. doi: 10.3390/vaccines13030331. Vaccines (Basel). 2025. PMID: 40266241 Free PMC article.
-
Generation of human MHC (HLA-A11/DR1) transgenic mice for vaccine evaluation.Hum Vaccin Immunother. 2016 Mar 3;12(3):829-36. doi: 10.1080/21645515.2015.1103405. Hum Vaccin Immunother. 2016. PMID: 26479036 Free PMC article.
-
Screening HLA-A-restricted T cell epitopes of SARS-CoV-2 and the induction of CD8+ T cell responses in HLA-A transgenic mice.Cell Mol Immunol. 2021 Dec;18(12):2588-2608. doi: 10.1038/s41423-021-00784-8. Epub 2021 Nov 2. Cell Mol Immunol. 2021. PMID: 34728796 Free PMC article.
-
Identification of novel HLA-A11-restricted T-cell epitopes in the Ebola virus nucleoprotein.Microbes Infect. 2019 Jan-Feb;21(1):56-62. doi: 10.1016/j.micinf.2018.04.005. Epub 2018 Oct 10. Microbes Infect. 2019. PMID: 29775667
-
Immunogenicity evaluation of a rationally designed polytope construct encoding HLA-A*0201 restricted epitopes derived from Leishmania major related proteins in HLA-A2/DR1 transgenic mice: steps toward polytope vaccine.PLoS One. 2014 Oct 13;9(10):e108848. doi: 10.1371/journal.pone.0108848. eCollection 2014. PLoS One. 2014. PMID: 25310094 Free PMC article.
References
-
- Zhang L., Kempf A., Nehlmeier I., Cossmann A., Richter A., Bdeir N., Graichen L., Moldenhauer A.S., Dopfer-Jablonka A., Stankov M.V., et al. SARS-CoV-2 BA.2.86 enters lung cells and evades neutralizing antibodies with high efficiency. Cell. 2024;187:596–608.E17. doi: 10.1016/j.cell.2023.12.025. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

