Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges
- PMID: 40333304
- PMCID: PMC12030821
- DOI: 10.3390/vaccines13040420
Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges
Abstract
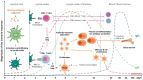
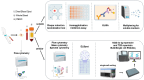
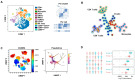
Monitoring the immune response to vaccination encompasses both significant challenges and promising opportunities for scientific advancement. The primary challenge lies in the inherent complexity and interindividual variability of immune responses, influenced by factors including age, genetic background, and prior immunological history. This variability necessitates the development of sophisticated, highly sensitive assays capable of accurately quantifying immune parameters such as antibody titers, T-cell responses, and cytokine profiles. Furthermore, the temporal dynamics of the immune response require comprehensive longitudinal studies to elucidate the durability and quality of vaccine-induced immunity. Challenges of this magnitude pave the way for immunological research advancements and diagnostic methodologies. Cutting-edge monitoring techniques, such as high-throughput sequencing and advanced flow cytometry, enable deeper insights into the mechanistic underpinnings of vaccine efficacy and contribute to the iterative design of more effective vaccines. Additionally, the integration of analytical tools holds the potential to predict immune responses and tailor personalized vaccination strategies. This will be addressed in this review to provide insight for enhancing public health outcomes and fortifying preparedness against future infectious disease threats.
Keywords: flow cytometry; immune response; reproducibility; vaccination.
Conflict of interest statement
A.L. and L.M. are both employed by Beckman Coulter Life Sciences.
Figures

Similar articles
-
Preexisting memory CD4 T cells in naïve individuals confer robust immunity upon hepatitis B vaccination.Elife. 2022 Jan 25;11:e68388. doi: 10.7554/eLife.68388. Elife. 2022. PMID: 35074048 Free PMC article.
-
Adaptive immune responses and cytokine immune profiles in humans following prime and boost vaccination with the SARS-CoV-2 CoronaVac vaccine.Virol J. 2022 Dec 22;19(1):223. doi: 10.1186/s12985-022-01957-1. Virol J. 2022. PMID: 36550578 Free PMC article.
-
Nanomedicines for an Enhanced Immunogenic Cell Death-Based In Situ Cancer Vaccination Response.Acc Chem Res. 2024 Mar 19;57(6):905-918. doi: 10.1021/acs.accounts.3c00771. Epub 2024 Feb 28. Acc Chem Res. 2024. PMID: 38417027
-
Methods for Measuring T-Cell Memory to Vaccination: From Mouse to Man.Vaccines (Basel). 2018 Jul 21;6(3):43. doi: 10.3390/vaccines6030043. Vaccines (Basel). 2018. PMID: 30037078 Free PMC article. Review.
-
Advances and Challenges in Aeromonas hydrophila Vaccine Development: Immunological Insights and Future Perspectives.Vaccines (Basel). 2025 Feb 18;13(2):202. doi: 10.3390/vaccines13020202. Vaccines (Basel). 2025. PMID: 40006748 Free PMC article. Review.
Cited by
-
Immunological Factors in Recurrent Pregnancy Loss: Mechanisms, Controversies, and Emerging Therapies.Biology (Basel). 2025 Jul 17;14(7):877. doi: 10.3390/biology14070877. Biology (Basel). 2025. PMID: 40723434 Free PMC article. Review.
References
-
- Marshall J.L. Testing vaccines: Essential steps in clinical trial design. In: Ashfield R., Nnamdi Oli A., Esimone C., Anagu L., editors. Vaccinology and Methods in Vaccine Research. Academic Press; Cambridge, MA, USA: 2022. pp. 295–310. Developments in Immunology.
Publication types
LinkOut - more resources
Full Text Sources

