Chronicling the Journey of Pneumococcal Conjugate Vaccine Introduction in India
- PMID: 40333310
- PMCID: PMC12031612
- DOI: 10.3390/vaccines13040432
Chronicling the Journey of Pneumococcal Conjugate Vaccine Introduction in India
Abstract
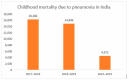
Background: Globally, pneumonia claims the lives of about 700,000 children under the age of 5 every year. Pneumococcal conjugate vaccine (PCV) was introduced in India phase-wise, beginning in high-burden states, and the rollout was completed nationwide by 2021-representing a major initiative by the Ministry of Health and Family Welfare (MoHFW). Despite the challenges posed by the COVID-19 pandemic, the campaign succeeded in maintaining progress and achieving nationwide coverage. This narrative review highlights the significant decisions, processes, and coordinated efforts of the various stakeholders involved that led to this successful PCV rollout.
Methodology: A comprehensive desk review of both published and unpublished literature relevant to pneumonia burden and the efficacy and effectiveness of PCVs, along with documentation of PCV introduction and the scale-up was carried out.
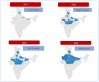
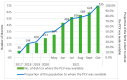
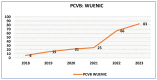
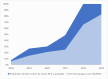
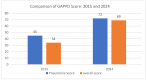
Results: The documentation of the PCV journey has been broken down into four sections: pre-introduction, PCV Phase-I introduction, pan-India rapid expansion, and the period post-introduction. Since the nationwide rollout in 2021, PCV coverage in India has steadily increased, reflecting successful immunization efforts. WUENIC, which is an annual WHO, and UNICEF estimates of national immunization coverage also show a positive trend in vaccination coverage (PCV booster coverage = 25% (2021), rising to 83% (2023), aligning with the goals of the WHO and UNICEF's Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD).
Conclusions: The phased rollout was an ambitious effort by the MoHFW, which was particularly challenging given the overlap with the COVID-19 pandemic. Despite these hurdles, the MoHFW, along with strong collaboration from development partners and stakeholders, successfully navigated the complex rollout. Future studies on the role of PCVs in reducing antibiotic resistance and the economic benefits of PCV introduction could help policymakers sustain funding and prioritize vaccine procurement decisions.
Keywords: COVID-19; pneumococcal conjugate vaccine; universal immunization program.
Conflict of interest statement
Pawan Kumar and Kapil Singh are employed at the Immunization Division, Ministry of Health and Family Welfare, Government of India. Abida Sultana, Rhythm Hora, Rashmi Mehra, Amanjot Kaur, Seema S. Koshal, Syed F. Quadri, Shyam Kumar Singh, and Arup Deb Roy were employed by the company John Snow India. Arindam Ray and Amrita Kumari were employed by the company Gates Foundation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- UNICEF. 2024. November. A Child Dies of Pneumonia Every 43 Seconds. [(accessed on 14 February 2025)]. Available online: https://data.unicef.org/topic/child-health/pneumonia.
-
- Ram S.V.D., Suman A.K. Prevention From Pneumonia. Government Of India Ministry Of Health And Family Welfare Department of Health and Family Welfare. 2023. [(accessed on 14 February 2025)]. Available online: https://sansad.in/getFile/loksabhaquestions/annex/1714/AU1004.pdf?source....
-
- O’Brien K.L., Wolfson L.J., Watt J.P., Henkle E., Deloria-Knoll M., McCall N., Lee E., Mulholland K., Levine O.S., Cherian T., et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

