Host-Pathogen Interaction Interface: Promising Candidate Targets for Vaccine-Induced Protective and Memory Immune Responses
- PMID: 40333316
- PMCID: PMC12031405
- DOI: 10.3390/vaccines13040418
Host-Pathogen Interaction Interface: Promising Candidate Targets for Vaccine-Induced Protective and Memory Immune Responses
Abstract
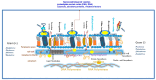
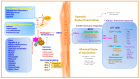
Vaccine formulations are a successful strategy against pathogen transmission because vaccine candidates induce effective and long-lasting memory immune responses (B and CD4+ T cells) at systemic and mucosal sites. Extracellular vesicles of lipoproteins, bioactive compounds from plants and invertebrates (sponges) encapsulated in liposomes, and glycoproteins can target these sites. The vaccine candidates developed can mimic microbial pathogens in a way that successfully links the innate and adaptive immune responses. In addition, vaccines plus adjuvants promote and maintain an inflammatory response. In this review, we aimed to identify the host-pathogen interface as a rich source of candidate targets for vaccine-induced protective and long-lasting memory immune responses.
Keywords: Gram-negative pathogenic bacteria; Gram-positive; adjuvants; cellular immune response; innate; prime boost; route of immunization; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Jeyanathan M., Vaseghi-Shanjani M., Afkhami S., Grondin J.A., Kang A., D’Agostino M.R., Yao Y., Jain S., Zganiacz A., Kroezen Z., et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat. Immunol. 2022;23:1687–1702. doi: 10.1038/s41590-022-01354-4. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

