Characterization of Cellular and Humoral Immunity to Commercial Cattle BVDV Vaccines in White-Tailed Deer
- PMID: 40333321
- PMCID: PMC12031561
- DOI: 10.3390/vaccines13040427
Characterization of Cellular and Humoral Immunity to Commercial Cattle BVDV Vaccines in White-Tailed Deer
Abstract
Background/objectives: White-tailed deer (Odocoileus virginianus) (WTD) play a central role at the human-livestock-wildlife interface, given their contribution to the spread of diseases that can affect livestock. These include a variety of bacterial, viral, and prion diseases with significant economic impact. Given the implications for WTD as potential reservoirs for a variety of diseases, methods for prevention and disease control in WTD are an important consideration.
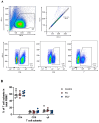
Methods: Using commercial livestock vaccines against bovine viral diarrhea virus (BVDV) in killed and modified live formulations, we test the ability of WTD to develop humoral and cellular immune responses following vaccination.
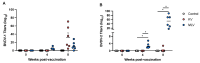
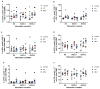
Results: We demonstrate that, similar to cattle, WTD develop humoral immune responses to both killed and modified live formulations.
Conclusions: As the farmed deer industry and the use of livestock vaccines in non-approved species grow, this type of information will help inform and develop improved husbandry and veterinary care practices. Additionally, while we were unable to detect cell-mediated immune responses to the vaccine, we established PrimeFlow as a method to detect IFN-γ responses in specific T cell populations, adding another level of resolution to our ability to understand WTD immune responses.
Keywords: BVDV; PrimeFlow; bovine viral diarrhea virus; immune responses; vaccines; white-tailed deer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prevalence and spatial distribution of antibodies to bovine viral diarrhea virus and Coxiella burnetii in white-tailed deer (Odocoileus virginianus) in New York and Pennsylvania.J Zoo Wildl Med. 2012 Sep;43(3):466-72. doi: 10.1638/2011-0049R.1. J Zoo Wildl Med. 2012. PMID: 23082509
-
Experimental infection of white-tailed deer fawns (Odocoileus virginianus) with bovine viral diarrhea virus type-1 isolated from free-ranging white-tailed deer.J Wildl Dis. 2009 Jul;45(3):653-60. doi: 10.7589/0090-3558-45.3.653. J Wildl Dis. 2009. PMID: 19617475
-
Bovine Viral Diarrhea Virus (BVDV) in White-Tailed Deer (Odocoileus virginianus).Front Microbiol. 2016 Jun 20;7:945. doi: 10.3389/fmicb.2016.00945. eCollection 2016. Front Microbiol. 2016. PMID: 27379074 Free PMC article. Review.
-
Measuring CMI responses using the PrimeFlow RNA assay: A new method of evaluating BVDV vaccination response in cattle.Vet Immunol Immunopathol. 2020 Mar;221:110024. doi: 10.1016/j.vetimm.2020.110024. Epub 2020 Feb 11. Vet Immunol Immunopathol. 2020. PMID: 32070831
-
Maternal antibody blocks humoral but not T cell responses to BVDV.Biologicals. 2003 Jun;31(2):123-5. doi: 10.1016/s1045-1056(03)00027-7. Biologicals. 2003. PMID: 12770543 Review.
References
-
- Campbell T.A., Vercauteren K.C. Diseases and Parasites. In: Hewitt D.G., editor. Biology and Management of White-Tailed Deer. CRC Press; Boca Raton, FL, USA: 2011. pp. 219–249.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

