Immunogenicity and Protective Efficacy of Five Vaccines Against Highly Pathogenic Avian Influenza Virus H5N1, Clade 2.3.4.4b, in Fattening Geese
- PMID: 40333332
- PMCID: PMC12031072
- DOI: 10.3390/vaccines13040399
Immunogenicity and Protective Efficacy of Five Vaccines Against Highly Pathogenic Avian Influenza Virus H5N1, Clade 2.3.4.4b, in Fattening Geese
Abstract
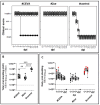
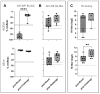
Background/Objectives: The risk of the introduction of highly pathogenic avian influenza virus (HPAIV) in geese breeding and fattening flocks is heightened due to the necessity of free-range access to grazing grounds. This study aimed to evaluate the safety, immunogenicity, and protective efficacy of five commercial vaccines against HPAIV subtype H5N1 (clade 2.3.4.4b) in subadult fattening geese. Methods: A prime-boost vaccination trial was conducted using five commercial vaccines, including H5 expressing vaccines of novel technology (subunit, vector, RNA) and whole inactivated virus (WIV) vaccines. Based on serological results, one RNA and one WIV vaccine were selected for a homologous challenge experiment. Results: Two vaccines of novel technology (vector, RNA) required a booster dose to raise specific antibodies titers above a threshold of four log2 using a hemagglutination inhibition (HI) assay, whereas a subunit vaccine and two WIV vaccines induced seroconversion after primary vaccination. In the challenge experiment, all unvaccinated control geese succumbed to infection by day four. In contrast, all vaccinated geese that had seroconverted exhibited full clinical protection. Although sterile immunity was not achieved, viral excretion was significantly reduced in the vaccinated groups compared to controls. Conclusions: Vaccination substantially mitigated the impact of HPAIV H5N1, clade 2.3.4.4b infection in geese, greatly improving animal welfare by preventing severe disease. Additionally, there was a significant reduction in viral burden. Further studies are necessary to verify the potential of these vaccines to reduce susceptibility to infection and virus excretion in order to achieve suppression of the between-flock reproduction number to < 1 in geese flocks at high risk of infection.
Keywords: H5N1; HPAIV; free range; goose; poultry; protection; vaccination.
Conflict of interest statement
Christophe Cazaban was employed by CEVA Santé Animale. Leticia Frizzo da Silva was employed bu Zoetis Inc. Heike Hufen was employed by Boehringer Ingelheim Vetmedica GmbH. The rest of the authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Hautefeuille C., Dauphin G., Peyre M. Knowledge and remaining gaps on the role of animal and human movements in the poultry production and trade networks in the global spread of avian influenza viruses—A scoping review. PLoS ONE. 2020;15:e0230567. doi: 10.1371/journal.pone.0230567. - DOI - PMC - PubMed
-
- Koopmans M.P.G., Behravesh C.B., Cunningham A.A., Adisasmito W.B., Almuhairi S., Bilivogui P., Bukachi S.A., Casas N., Becerra N.C., Charron D.F., et al. The panzootic spread of highly pathogenic avian influenza H5N1 sublineage 2.3.4.4b: A critical appraisal of One Health preparedness and prevention. Lancet Infect. Dis. 2024;24:e774–e781. doi: 10.1016/S1473-3099(24)00438-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

