Modern Catalytic Materials for the Oxygen Evolution Reaction
- PMID: 40333588
- PMCID: PMC12029354
- DOI: 10.3390/molecules30081656
Modern Catalytic Materials for the Oxygen Evolution Reaction
Abstract
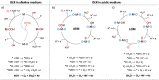
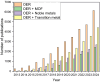
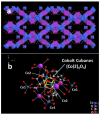
The oxygen evolution reaction (OER) has, in recent years, attracted great interest from scientists because of its prime role in a number of renewable energy technologies. It is one of the reactions that occurs during hydrogen production through water splitting, is used in rechargeable metal-air batteries, and plays a fundamental role in regenerative fuel cells. Therefore, there is an emerging need to develop new, active, stable, and cost-effective materials for OER. This review presents the latest research on various groups of materials, showing their potential to be used as OER electrocatalysts, as well as their shortcomings. Particular attention has been paid to metal-organic frameworks (MOFs) and their derivatives, as those materials offer coordinatively unsaturated sites, high density of transition metals, adjustable pore size, developed surface area, and the possibility to be modified and combined with other materials.
Keywords: metal–organic frameworks; noble metals; oxygen evolution reaction; transition oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
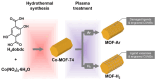



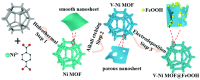
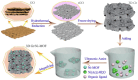

References
-
- Tahir M., Pan L., Idrees F., Zhang X., Wang L., Zou J.J., Wang Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy. 2017;37:136–157. doi: 10.1016/j.nanoen.2017.05.022. - DOI
-
- Carmo M., Fritz D.L., Mergel J., Stolten D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy. 2013;38:4901–4934.
-
- Wei C., Rao R.R., Peng J., Huang B., Stephens I.E.L., Risch M., Xu Z.J., Shao-Horn Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019;31:1806296. - PubMed
-
- Zhang L., Zhao H., Xu S., Liu Q., Li T., Luo Y., Gao S., Shi X., Asiri A.M., Sun X. Recent Advances in 1D Electrospun Nanocatalysts for Electrochemical Water Splitting. Small Struct. 2021;2:2000048.
Publication types
LinkOut - more resources
Full Text Sources

