Phytotoxicity, Cytotoxicity, and Antimicrobial Activity of Triethanolammonium Amino Acids Salts
- PMID: 40333600
- PMCID: PMC12029836
- DOI: 10.3390/molecules30081712
Phytotoxicity, Cytotoxicity, and Antimicrobial Activity of Triethanolammonium Amino Acids Salts
Abstract
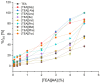
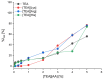
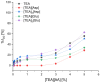
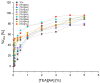
The growing use of ionic liquids (ILs) necessitates an understanding of their environmental impact and toxicity levels. In this study, a series of amino acid-based ionic liquids containing the triethanolammonium (TEA) cation were evaluated for their biological activity against Lepidium sativum L., the mouse fibroblast cell line L929, a selection of gram-positive and gram-negative bacteria, and the yeast Candida albicans. The influence of amino acid anion structure on toxicity was also examined. Among the tested ionic liquids, [TEA][Asp] exhibited low toxicity toward Lepidium sativum L., representing terrestrial plants, while [TEA][Phe] showed the lowest cytotoxicity. Regarding microbial activity, [TEA][Lys] demonstrated greater bactericidal effectiveness against E. coli than S. aureus, while both [TEA][Lys] and [TEA][Arg] exhibited the strongest inhibitory effect against C. albicans. Our findings underscore the crucial role of IL salt composition in determining biological activity, highlighting the significance of interactions between IL components in shaping their potential effects.
Keywords: amino acids ionic liquids; antimicrobial activity; cytotoxicity; phytotoxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





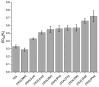
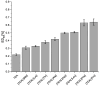
References
-
- Singh P., Rajkhowa S., Sen A., Sarma J., editors. Handbook of Ionic Liquids: Fundamentals, Applications, and Sustainability. 1st ed. Wiley; Hoboken, NJ, USA: 2024.
-
- Sawant A.D., Raut D.G., Darvatkar N.B., Salunkhe M.M. Recent Developments of Task-Specific Ionic Liquids in Organic Synthesis. Green Chem. Lett. Rev. 2011;4:41–54. doi: 10.1080/17518253.2010.500622. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

