Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from 'In Vitro' Studies
- PMID: 40333601
- PMCID: PMC12029976
- DOI: 10.3390/molecules30081684
Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from 'In Vitro' Studies
Abstract
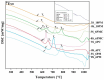
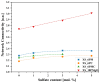
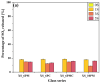
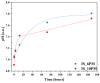
Vitreous carriers of essential nutrients should release elements in response to plant demand, minimizing over-fertilization risks. This study focused on designing and characterizing sulfate-bearing slow-release fertilizers based on four glass series (41SiO2∙6(10)P2O5∙20K2O-33(29)MgO/CaO/MgO + CaO) with increasing sulfate content. Structural analysis identified a network dominated by QSi2 units, with some QSi3 species and isolated QP0 units. This fragmented structure resulted in high solubility in acidic environments while maintaining water resistance. Such dual behavior is a direct consequence of the delicate balance between depolymerized silicate chains and isolated orthophosphate units, which ensure rapid ion exchange under acidic conditions while preventing uncontrolled leaching in neutral media. Nutrient leaching depended on SO3 content, affecting matrix rigidity, and on the type of alkaline earth modifier and P2O5 content. Dissolution kinetics showed an initial rapid release phase, followed by stabilization governed by silicate hydrolysis. Thermal analysis linked network flexibility to dissolution behavior-CaO promoted an open structure with high SiO2 release, MgO increased rigidity, while their co-addition reduced ion diffusion and silica dissolution. The thermal behavior of the glasses provided indirect insight into their structural rigidity, revealing how compositional variations influence the mechanical stability of the network. This structural rigidity, inferred from glass transition and crystallization phenomena, was found to correlate with the selective dissolution profiles observed in acidic versus neutral environments. These results reveal complex interactions between composition, structure, and nutrient release, shaping the agricultural potential of these glasses.
Keywords: dissolution kinetics; glass structure; nutrient leaching; slow-release fertilizers; sulfate-bearing glasses.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
















References
-
- Fiantis D., Ginting F.I., Gusnidar, Nelson M., Minasny B. Volcanic Ash, insecurity for the people but securing fertile soil for the future. Sustainability. 2019;11:3072. doi: 10.3390/su11113072. - DOI
-
- Neall V.E. Encyclopaedia of Life Support Systems. Volume 1. UNESCO; Paris, France: 2006. Land use and land cover; pp. 1–24.
-
- Stoch L., Wacławska I., Szumera M. Phospho-silicate glasses, self-degradable in environment conditions. Adv. Mater. Res. 2008;39–40:335–340. doi: 10.4028/www.scientific.net/AMR.39-40.335. - DOI
-
- Dakora F.D., Phillips D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 2002;245:35–47. doi: 10.1023/A:1020809400075. - DOI
-
- Vega N.W.O. A review on beneficial effects of Rhizosphere bacteria on soil nutrient availability and plant nutrient uptake. Nac. Agron.-Medellín. 2007;60:3621–3643.
Grants and funding
LinkOut - more resources
Full Text Sources

