Delineating Host-Guest-Solvent Interactions in Solution from Gas-Phase Host-Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase
- PMID: 40333634
- PMCID: PMC12029154
- DOI: 10.3390/molecules30081723
Delineating Host-Guest-Solvent Interactions in Solution from Gas-Phase Host-Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase
Abstract
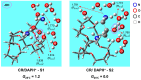
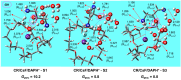
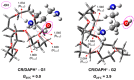
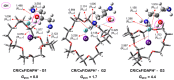
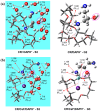
We study the structures of 24-crown-8/H+/diaminopropanol (CR/DAPH+) and 24-crown-8/CsF/H+/diaminopropanol (CR/CsF/DAPH+) non-covalent host-guest complexes in both the gas phase and aqueous solution using the density functional theory (DFT) method. We examine the environment (complexation with CR vs. solvation) around the guest functional groups (ammoium, hydroxyl, and amino) in the CR/DAPH+ and CR/CsF/DAPH+ complexes. We find that the gas-phase configurations with the 'naked' hydroxyl/amino devoid of H-bonding with CR or CR/CsF are structurally correlated with the lowest Gibbs free energy conformers in aqueous solution in which the functional groups are solvated off the CR or CR/CsF host. We predict that the latter thermodynamically disadvantageous host-guest configurations would be identified in the gas phase by infrared multiphoton dissociation (IRMPD) spectroscopy, originating from the complexes in aqueous solution. This predicted 'thermodynamic reversal' and 'structural correlation' of the host-guest configurations in the gas phase vs. in solution are discussed in relation to the possibility of obtaining information on host-guest-solvent interactions in the solution phase from the gas-phase host-guest configurations.
Keywords: crown ether; diaminopropanol; host–guest; structural correlation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Schalley C.A. Supramolecular chemistry goes gas phase: The mass spectrometric examination of noncovalent interactions in host–guest chemistry and molecular recognition. Int. J. Mass Spectrom. 2000;194:11–39. doi: 10.1016/S1387-3806(99)00243-2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

