Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior
- PMID: 40333645
- PMCID: PMC12029862
- DOI: 10.3390/molecules30081719
Amphiphilic Styrene-Based Pyrene Derivatives: Tunable Aggregation Luminescence and Their Photo-Induced Dimerization Behavior
Abstract
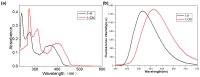
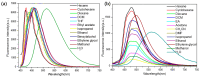
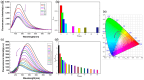
Since the discovery of the aggregation-induced emission (AIE) phenomenon, various stimuli-responsive materials have been rapidly developed. However, how to achieve the transition between aggregation-caused quenching (ACQ) and AIE through molecular design is an urgent problem to be solved. In this work, we synthesized and studied the aggregation luminescence behavior and photochromism of two different substituted pyrene ethylene derivatives, 1-H and 1-CN. Due to the different substituents attached to the ethylene unit, 1-H exhibits ACQ luminescence behavior. When the substituent is a cyanide group, it exhibits AIE behavior. In addition, the ordered nanoparticles formed by self-assembly in aqueous solution exhibit interesting photo-induced cyclization behavior, which leads to fluorescence quenching under ultraviolet light irradiation (λ = 365 nm). Therefore, due to their amphiphilicity and photo-responsiveness, these compounds can be used as anticounterfeiting inks in information encryption. This work contributes new members to the family of amphiphilic photo-responsive materials and demonstrates their potential applications in optical information storage and multi-color luminescence.
Keywords: aggregation-induced emission; luminescent material; photochemical reaction; pyrene; self-assembly.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Thermosensitive Microgels Containing AIEgens: Enhanced Luminescence and Distinctive Photochromism for Dynamic Anticounterfeiting.ACS Appl Mater Interfaces. 2022 Apr 20;14(15):17794-17805. doi: 10.1021/acsami.2c01620. Epub 2022 Apr 11. ACS Appl Mater Interfaces. 2022. PMID: 35404060
-
Multicolor-Luminescence Including White Light by Photomodulation of Supramolecular Assemblies in Aqueous Media.ACS Appl Mater Interfaces. 2022 Aug 17;14(32):36936-36946. doi: 10.1021/acsami.2c07836. Epub 2022 Aug 3. ACS Appl Mater Interfaces. 2022. PMID: 35919994
-
Aggregation behaviour of pyrene-based luminescent materials, from molecular design and optical properties to application.Chem Soc Rev. 2023 Oct 2;52(19):6715-6753. doi: 10.1039/d3cs00251a. Chem Soc Rev. 2023. PMID: 37694728 Review.
-
Polymorphism and Light-Driven Forward Movement of TPE Derivative Micro-Crystal due to ArH-pi Interactions Difference.Chemistry. 2023 Dec 6;29(68):e202302567. doi: 10.1002/chem.202302567. Epub 2023 Oct 20. Chemistry. 2023. PMID: 37709727
-
Self-Assembly of Aggregation-Induced-Emission Molecules.Chem Asian J. 2019 Mar 15;14(6):730-750. doi: 10.1002/asia.201801884. Epub 2019 Mar 6. Chem Asian J. 2019. PMID: 30839162 Review.
References
-
- Cao X., Gao A., Hou J., Yi T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021;434:213792. doi: 10.1016/j.ccr.2021.213792. - DOI
-
- Xu Q., Qin Z., Bei Y., Feng S., Xu X. A cationic amphiphilic tetraphenylethylene derivative with hydrochromic sensitive property: Applications in anti-counterfeiting ink and rewritable paper. Chin. Chem. Lett. 2022;33:4838–4841. doi: 10.1016/j.cclet.2022.01.079. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

