AAV delivery of GBA1 suppresses α-synuclein accumulation in Parkinson's disease models and restores functions in Gaucher's disease models
- PMID: 40333681
- PMCID: PMC12057913
- DOI: 10.1371/journal.pone.0321145
AAV delivery of GBA1 suppresses α-synuclein accumulation in Parkinson's disease models and restores functions in Gaucher's disease models
Abstract
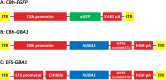
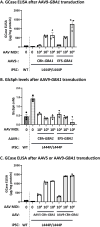
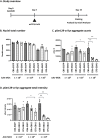
Biallelic mutations in the glucosylceramidase beta 1 (GBA1) gene are the underlying genetic cause of Gaucher's disease (GD), resulting in a deficient lysosomal hydrolase and subsequent accumulation of glycosphingolipids. More recently, GBA1 mutations have been identified as the most prevalent genetic risk factor for Parkinson's disease (PD), associated with more pronounced symptoms characterized by earlier onset and accelerated cognitive decline. In these GBA-associated PD patients the α-synuclein pathology is more prominent, and recent data suggest a link between α-synucleinopathies and GBA1 mutations. Here, we explored the effect of GBA1 gene supplementation on the GD phenotypes and α-synuclein pathology by using the adeno-associated virus (AAV) system. We have compared two AAV serotypes, AAV5 and AAV9, and two different ubiquitous promoters, and demonstrate that both promoters work efficiently albeit not the same in vitro and in vivo. GBA1 overexpression reduces the accumulation of glucosylsphingosine (GlcSph) and restores motor dysfunction in a GD mouse model. We further demonstrate that GBA1 overexpression can dissolve phospho-α-synuclein aggregation induced by the addition of α-synuclein pre-formed fibril (PFF) in a mouse primary neuron model suggesting the direct effect of β-Glucocerebrosidase (GCase) on α-synuclein accumulation. In vivo, we show that GCase inhibition can induce insoluble high-molecular-weight α-synuclein aggregation and that delivery of GBA1 achieves robust reduction of the α-synuclein aggregates in the mouse brain. In summary, GCase expression not only reduces GlcSph, but also restores GD motor dysfunction and removes α-synuclein aggregates which are the hallmark for PD and α-synucleinopathies. AAV delivery of GBA1 is a powerful approach to restore glucocerebrosidase function and to resolve misfolded α-synuclein protein, with applications for GD and PD.
Copyright: © 2025 Okai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: T. Okai, S. Sato, S. Matsumoto, M. Nakayama, S. Yamamoto, T. Hioki, and M. Tanaka report on a relationship with Takeda Pharmaceutical Company Limited that includes employment and non-financial support. M. Deshpande, B. Strack-Logue and G. Proetzel report on a relationship with Takeda Development Center Americas that includes employment and non-financial support.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

