Tumor Microenvironment Lactate: Is It a Cancer Progression Marker, Immunosuppressant, and Therapeutic Target?
- PMID: 40333742
- PMCID: PMC12029365
- DOI: 10.3390/molecules30081763
Tumor Microenvironment Lactate: Is It a Cancer Progression Marker, Immunosuppressant, and Therapeutic Target?
Abstract
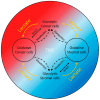
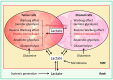
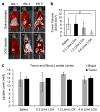
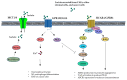
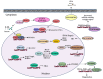
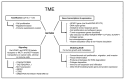
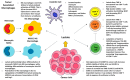
The "Warburg effect" is a term coined a century ago for the preferential use of glycolysis over aerobic respiration in tumor cells for energy production, even under aerobic conditions. Although this is a less efficient mechanism of generating energy from glucose, aerobic glycolysis, in addition to the canonical anaerobic glycolysis, is an effective means of lactate production. The abundant waste product, lactate, yielded by the dual glycolysis in a tumor, has been discovered to be a major biomolecule that drives cancer progression. Lactate is a metabolic energy source that, via cell membrane lactate transporters, shuttles in and out of cancer cells as well as cancer cell-associated stromal cells and immune cells within the tumor microenvironment (TME). Additionally, lactate serves as a pH tuner, signaling ligand and transducer, epigenetic and gene transcription regulator, TME modifier, immune suppressor, chemoresistance modulator, and prognostic marker. With such broad functionalities, the production-consumption-reproduction of TME lactate fuels tumor growth and dissemination. Here, we elaborate on the lactate sources that contribute to the pool of lactate in the TME, the functions of TME lactate, the influence of the TME lactate on immune cell function and local tissue immunity, and anticancer therapeutic approaches adopting lactate manipulations and their efficacies. By scrutinizing these properties of the TME lactate and others that have been well addressed in the field, it is expected that a better weighing of the influence of the TME lactate on cancer development, progression, prognosis, and therapeutic efficacy can be achieved.
Keywords: cell metabolism; immune regulation; lactate; posttranslational modification; signaling and gene regulation; tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Research progress on the regulatory role of lactate and lactylation in tumor microenvironment.Biochim Biophys Acta Rev Cancer. 2025 Jul;1880(3):189339. doi: 10.1016/j.bbcan.2025.189339. Epub 2025 Apr 29. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40311713 Review.
-
Lactylation in cancer progression and drug resistance.Drug Resist Updat. 2025 Jul;81:101248. doi: 10.1016/j.drup.2025.101248. Epub 2025 Apr 21. Drug Resist Updat. 2025. PMID: 40287994 Review.
-
Lactate-mediated metabolic reprogramming of tumor-associated macrophages: implications for tumor progression and therapeutic potential.Front Immunol. 2025 May 13;16:1573039. doi: 10.3389/fimmu.2025.1573039. eCollection 2025. Front Immunol. 2025. PMID: 40433363 Free PMC article. Review.
-
Lactate in the tumour microenvironment: From immune modulation to therapy.EBioMedicine. 2021 Nov;73:103627. doi: 10.1016/j.ebiom.2021.103627. Epub 2021 Oct 14. EBioMedicine. 2021. PMID: 34656878 Free PMC article. Review.
-
Lactate and lactylation: Behind the development of tumors.Cancer Lett. 2024 Jun 1;591:216896. doi: 10.1016/j.canlet.2024.216896. Epub 2024 Apr 17. Cancer Lett. 2024. PMID: 38641309 Review.
References
-
- Warburg O. The Metabolism of Carcinoma Cells. Cancer Res. 1925;9:148–163. doi: 10.1158/jcr.1925.148. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

