Performance of BIOCREDIT Pf/Pv lactate dehydrogenase-based malaria rapid diagnostic test among pregnant women with suspected malaria infection in Bahir Dar City Administration, northwest Ethiopia
- PMID: 40333746
- PMCID: PMC12057965
- DOI: 10.1371/journal.pone.0322362
Performance of BIOCREDIT Pf/Pv lactate dehydrogenase-based malaria rapid diagnostic test among pregnant women with suspected malaria infection in Bahir Dar City Administration, northwest Ethiopia
Abstract
Background: Malaria during pregnancy is a common public health problem in sub-Saharan Africa. It poses a double burden as it affects the health of mothers, their fetuses and neonates. Moreover, due to malaria parasites sequestration in the placenta, microscopy might miss infections. Hence, rapid diagnostic tests (RDTs) are good alternatives for diagnosing malaria in pregnancy even at health facilities or periphery or lower-level healthcare facilities (e.g., health posts). However, performance of RDTs should be closely monitored as their sensitivity and specificity are affected by many factors.
Methods: A health facility-based cross-sectional study was conducted among 302 pregnant women with suspected malaria infection to evaluate the performance of the newly introduced BIOCREDIT Pf/Pv plasmodial lactate dehydrogenase (pLDH) RDT. Venous blood samples were collected from all eligible pregnant women and tested for Plasmodium infection using BIOCREDIT Pf/Pv and CareStart™ Pf/Pv RDTs, microscopy and polymerase chain reaction (PCR) following standard protocols. The performance of BIOCREDIT Pf/Pv was evaluated using the following parameters: sensitivity, specificity, positive and negative predictive values and kappa-value. These parameters were calculated using online SISA software.
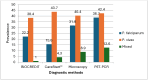
Results: Of the 302 pregnant women with complete data, 166 (55.0%), 180 (59.6%), 191 (63.2%) and 207 (68.5%) tested positive by CareStart™ Pf/Pv, BIOCREDIT Pf/Pv, microscopy and PCR, respectively. The sensitivity of BIOCREDIT Pf/Pv in detecting P. falciparum was 89.4%, 75.4% and 55.6% as compared to CareStart™ Pf/Pv, microscopy and PCR tests, respectively, while the sensitivity for P. vivax was 81.8%, 84.3% and 86.7%, respectively. The specificity of BIOCREDIT Pf/Pv was > 90% when compared to all the three diagnostic tests. When considering PCR as a reference test, BIOCREDIT Pf/Pv was more sensitive (55.6%) than CareStart™ Pf/Pv (37.6%) for detecting P. falciparum but had similar sensitivity (86.7%) in detecting P. vivax.
Conclusions: BIOCREDIT Pf/Pv performed better for the diagnosis of P. falciparum infection in pregnant women than the previously in-use CareStart™ Pf/Pv. We recommend using the BIOCREDIT Pf/Pv RDT in Ethiopia for the diagnosis of both species given the high prevalence and widespread nature of the hrp2/3 gene deletion in the country.
Copyright: © 2025 Tegegne et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures
References
-
- Federal Ministry of Health (FMOH). National Malaria Guidelines. 5th ed. Addis Ababa, Ethiopia; 2022.
-
- World Health Organization. Global Messaging Briefing Kit: World Malaria Report; 2023, [cited 20 Dec 2024]. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
-
- World Health Organization. World malaria report. 2023, [cited 23 Sep 2024]. Available from: https://www.mmv.org/sites/default/files/content/document/world-malaria-r...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical