Starting strong: development and biomechanics of the seedling-host interaction in European mistletoe (Viscum album)
- PMID: 40333818
- PMCID: PMC12369475
- DOI: 10.1093/jxb/eraf129
Starting strong: development and biomechanics of the seedling-host interaction in European mistletoe (Viscum album)
Abstract
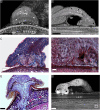
Attachment to a substrate is fundamental for plant growth and development. This is especially true for species that live either partially or fully off the ground, such as mistletoes, which have developed unique adaptations to anchor themselves securely to host trees from which they draw water and some nutrients. While the mechanical properties of attachment during the adult stages in many plant species have been described, the mechanical principles of the initial developmental stages are rarely investigated. Here, we focus on the parasitic European mistletoe (Viscum album L.) and its attachment to a host plant at the seedling stage. Using a combination of germination experiments, microtomography, histological analysis, and biomechanical tests, this work investigates the role of the three key attachment structures involved in this process: the seed coat, hypocotyl, and holdfast. The viscin layer, a sticky coating on the seed, provides initial adhesion before the growing hypocotyl expands towards the host surface, where it flattens and forms a holdfast that strengthens adhesion and aids tissue penetration. Tensile tests revealed that these three attachment structures withstand similar forces in the early stages, considerably higher than the weight of the seedling. Within a few months, the endophytic system interlocked with the host bark, forming a robust connection that not only transports water but also increased the mechanical strength of the structure. This work highlights the fundamental mechanisms of the initial mistletoe-host interaction, which forms the basis of their decades-long relationship.
Keywords: Haustorium; Santalaceae; microtomography; mistletoe; parasitic plant; plant biomechanics; seed attachment; seedling development; tensile test.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Loss of olfaction reduces caterpillar performance and increases susceptibility to a natural enemy.Elife. 2025 Aug 1;14:RP105585. doi: 10.7554/eLife.105585. Elife. 2025. PMID: 40747879 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Secure but flexible: temporary adhesion in mistletoe seedlings.J Exp Bot. 2025 Aug 21;76(12):3247-3251. doi: 10.1093/jxb/eraf205. J Exp Bot. 2025. PMID: 40663040 Free PMC article.
References
-
- Albert M, Belastegui-Macadam X, Kaldenhoff R.. 2006. An attack of the plant parasite Cuscuta reflexa induces the expression of attAGP, an attachment protein of the host tomato. The Plant Journal 48, 548–556. - PubMed
-
- Ang SLP, Yong JWH.. 2005. A protocol for in vitro germination and sustainable growth of two tropical mistletoes. Plant Cell, Tissue and Organ Culture 80, 221–228.
-
- Aukema JE. 2003. Vectors, viscin, and Viscaceae: mistletoes as parasites, mutualists, and resources. Frontiers in Ecology and the Environment 1, 212–219.
-
- Barney CW, Hawksworth FG, Geils BW.. 1998. Hosts of Viscum album. European Journal of Forest Pathology 28, 187–208.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

