A cross-sectional study on the endorsement of reporting guidelines and clinical trial registration among immunology and allergy journals
- PMID: 40333833
- PMCID: PMC12057991
- DOI: 10.1371/journal.pone.0322003
A cross-sectional study on the endorsement of reporting guidelines and clinical trial registration among immunology and allergy journals
Abstract
Background: Healthcare practitioners rely on research based on solid evidence for their clinical decisions, ensuring the provision of safe and effective patient care. The use of reporting guidelines and the registration of clinical trials enhance the reliability and credibility of research findings by promoting transparency and minimizing potential biases. However, it remains uncertain to what extent leading immunology and allergy journals have embraced these tools. This study aims to evaluate how commonly reporting guidelines and clinical trial registration are required and endorsed within leading immunology and allergy journals.
Methods: We identified the top 100 journals in the subcategory of "Immunology and Allergy" using the Scopus CiteScore tool for the year 2021. We thoroughly reviewed the "Instructions for Authors" section of each journal, focusing on indications related to specific reporting guidelines as outlined by the Enhancing the Quality and Transparency of Health Research (EQUATOR) Network, as well as the practice of clinical trial registration. Our documentation categorized statements as "Not Mentioned," "Recommended," "Not Accepted," or "Required." The category "Not Accepted" specifically indicated that the journal explicitly did not accept the study designs associated with certain reporting guidelines, rather than implying bias against these guidelines. ensure equitable evaluation, we communicated with each journal to confirm the types of articles they accepted.
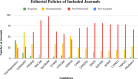
Results: Among the 100 journals assessed, the CONSORT guideline emerged as the most frequently cited, with 60 journals recommending adherence and 13 requiring it. Conversely, the QUOROM guideline was the least commonly cited, with merely two journals recommending its adherence and none requiring it. Nineteen journals did not reference a single reporting guideline. Remarkably, clinical trial registration was required by 42 journals and recommended by 34.
Conclusion: This study reveals variation in the adoption of reporting guidelines and clinical trial registration in immunology and allergy journals. While some journals strongly advocate for or require these practices, others do not emphasize them at all. This inconsistency affects research rigor and reproducibility, highlighting the need for stricter enforcement. Editors should encourage these practices to enhance transparency and minimize biases.
Copyright: © 2025 Khan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Genuneit J, Seibold AM, Apfelbacher CJ, Christian J. Apfelbacher, George NK. Overview of systematic reviews in allergy epidemiology. Allergy. 2017;72(6):849–856. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources