Global Reaction Route Mapping of C3H2O: Isomerization Pathways, Dissociation Channels, and Bimolecular Reaction with a Water Molecule
- PMID: 40333843
- PMCID: PMC12029752
- DOI: 10.3390/molecules30081829
Global Reaction Route Mapping of C3H2O: Isomerization Pathways, Dissociation Channels, and Bimolecular Reaction with a Water Molecule
Abstract
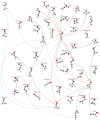
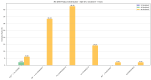
A comprehensive theoretical investigation of the C3H2O potential energy surface (PES) was conducted, revealing 30 equilibrium structures (EQs), 128 transition state structures (TSs), and 35 direct dissociation channels (DCs), establishing a global reaction network comprising 101 isomerization pathways and dissociation channels. Particular focus was placed on the five most stable isomers, H2CCCO (EQ3), OC(H)CCH (EQ7), H-c-CC(O)C-H (EQ0), HCC(H)CO (EQ1), and HO-c-CCC-H (EQ12), and their reactions with water molecules. Multicomponent artificial force-induced reaction (MC-AFIR) calculations were employed to study bimolecular collisions between H2O and these stable isomers. The product distributions revealed isomer-specific reactivity patterns: EQ3 and EQ7 predominantly formed neutral species at high collision energies, EQ0 produced both ionic and neutral species, while EQ1 and EQ12 exhibited more accessible reaction pathways at lower collision energies with a propensity for spontaneous isomerization. Born-Oppenheimer Molecular Dynamics (BOMD) simulations complemented these findings, suggesting several viable products emerge from reactions with water molecules, including HCCC(OH)2H (EQ7 + H2O), OCCHCH2OH (EQ1 + H2O), and HO-c-CC(H)C(OH)-H (EQ12 + H2O). This investigation elucidates the intrinsic relationships between isomers and their potential products, formed through biomolecular collisions with water molecules, establishing a fundamental framework for future conformational and reactivity studies of the C3H2O family.
Keywords: C3H2O; bimolecular reactions; conformational explorations; dissociation pathways; isomerization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Zamirri L., Ugliengo P., Ceccarelli C., Rimola A. Quantum Mechanical Investigations on the Formation of Complex Organic Molecules on Interstellar Ice Mantles: Review and Perspectives. ACS Earth Space Chem. 2019;3:1499–1523. doi: 10.1021/acsearthspacechem.9b00082. - DOI
-
- McGuire B.A. 2021 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astrophys. J. Suppl. Ser. 2022;259:30. doi: 10.3847/1538-4365/ac2a48. - DOI
-
- Guélin M., Cernicharo J. Organic Molecules in Interstellar Space: Latest Advances. Front. Astron. Space Sci. 2022;9:787567. doi: 10.3389/fspas.2022.787567. - DOI
-
- Puzzarini C., Alessandrini S., Bizzocchi L., Melosso M., Rivilla V.M. From the Laboratory to the Interstellar Medium: A Strategy to Search for Exotic Molecules in Space. Front. Astron. Space Sci. 2023;10:1211784. doi: 10.3389/fspas.2023.1211784. - DOI
-
- Herbst E. Unusual Chemical Processes in Interstellar Chemistry: Past and Present. Front. Astron. Space Sci. 2021;8:776942. doi: 10.3389/fspas.2021.776942. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

