Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil-Water Emulsion Under Gravity
- PMID: 40333854
- PMCID: PMC12029613
- DOI: 10.3390/molecules30081840
Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil-Water Emulsion Under Gravity
Abstract
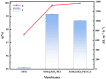
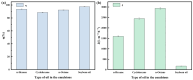
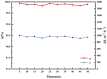
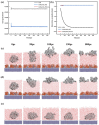
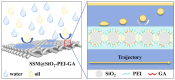
The separation of high-viscosity oil-water emulsions remains a global challenge due to ultra-stable interfaces and severe membrane fouling. In this paper, SiO2 micro-nanoparticles coated with polyethyleneimine (PEI) were initially loaded onto a stainless steel substrate. This dual-functional design simultaneously modifies surface roughness and wettability. Furthermore, a covalent crosslinking network was created through the Schiff base reaction between PEI and glutaraldehyde (GA) to enhance the stability of the membrane. The membrane exhibits extreme wettability, superhydrophilicity (WCA = 0°), and underwater superoleophobicity (UWOCA = 156.9°), enabling a gravity-driven separation of pump oil emulsions with 99.9% efficiency and a flux of 1006 L·m-2·h-1. Moreover, molecular dynamics (MD) simulations demonstrate that the SiO2-PEI-GA-modified membrane promotes the formation of a stable hydration layer, reduces the oil-layer interaction energy by 85.54%, and exhibits superior underwater oleophobicity compared to the unmodified SSM. Efficiency is maintained at 99.8% after 10 cycles. This study provides a scalable strategy that combines covalent crosslinking with hydrophilic particle modification, effectively addressing the trade-off between separation performance and membrane longevity in the treatment of viscous emulsions.
Keywords: crosslinking; emulsion separation; molecular dynamics simulation; superwetting membrane; underwater superoleophobic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Facile preparation of marine carrageenan hydrogel-coated steel mesh with superhydrophilic and underwater superoleophobic performance for highly efficient oil-water separation.Water Environ Res. 2025 Jan;97(1):e70006. doi: 10.1002/wer.70006. Water Environ Res. 2025. PMID: 39806543
-
"Biomass to Membrane": Sulfonated Kraft Lignin/PCL Superhydrophilic Electrospun Membrane for Gravity-Driven Oil-in-Water Emulsion Separation.ACS Appl Mater Interfaces. 2023 Sep 6;15(35):41961-41976. doi: 10.1021/acsami.3c09964. Epub 2023 Aug 25. ACS Appl Mater Interfaces. 2023. PMID: 37624730
-
Ultra-high flux mesh membranes coated with tannic acid-ZIF-8@MXene composites for efficient oil-water separation.Environ Res. 2024 May 1;248:118264. doi: 10.1016/j.envres.2024.118264. Epub 2024 Jan 23. Environ Res. 2024. PMID: 38266894
-
The separation of oily water using low-cost natural materials: Review and development.Chemosphere. 2021 Dec;285:131398. doi: 10.1016/j.chemosphere.2021.131398. Epub 2021 Jul 6. Chemosphere. 2021. PMID: 34252813 Review.
-
Strategies to modulate underwater oil wettability and adhesion.Adv Colloid Interface Sci. 2025 Jun;340:103442. doi: 10.1016/j.cis.2025.103442. Epub 2025 Feb 11. Adv Colloid Interface Sci. 2025. PMID: 39985951 Review.
References
-
- Huang W., Zhang L., Lai X., Li H., Zeng X. Highly hydrophobic F-rGO@wood sponge for efficient clean-up of viscous crude oil. Chem. Eng. J. 2020;386:123994. doi: 10.1016/j.cej.2019.123994. - DOI
-
- Wang W., Li Z., Chen C., Wei Y., Xu X., Liu H., Kuang C., Yang G., Li X., Qing Y., et al. Multifunctional superhydrophobic coating constructed from rosin-based polymer and nano-boehmite particles for oil-water separation, flame retardancy and anti-icing. Prog. Org. Coat. 2025;198:108872. doi: 10.1016/j.porgcoat.2024.108872. - DOI
-
- Fan Q., Lu T., Deng Y., Zhang Y., Ma W., Xiong R., Huang C. Bio-based materials with special wettability for oil-water separation. Sep. Purif. Technol. 2022;297:121445. doi: 10.1016/j.seppur.2022.121445. - DOI
-
- Xue Z., Cao Y., Liu N., Feng L., Jiang L. Special wettable materials for oil/water separation. J. Mater. Chem. A. 2014;2:2445–2460. doi: 10.1039/C3TA13397D. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

