The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers
- PMID: 40333859
- PMCID: PMC12029860
- DOI: 10.3390/molecules30081852
The Application of 2D Graphitic Carbon Nitride (g-C3N4) and Hexagonal Boron Nitride (h-BN) in Low-Temperature Fuel Cells: Catalyst Supports, ORR Catalysts, and Membrane Fillers
Abstract
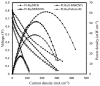
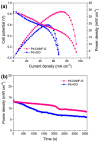
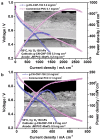
In recent years, two-dimensional (2D) graphitic carbon nitride (g-C3N4) and hexagonal boron nitride (h-BN) have gained remarkable attention due to their resemblance to graphene. These materials have a wide range of applications in energy and other sustainable fields, including heterogeneous catalysis and photocatalysis. g-C3N4 and h-BN can play different roles in low-temperature fuel cells. They can be used as catalyst supports, catalysts for oxygen reduction, and membrane fillers. In this work, the application of pure and doped g-C3N4 and h-BN, alone or as composite materials, in low-temperature fuel cells is overviewed.
Keywords: catalysts; fuel cells; g-C3N4; h-BN; polymer membranes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Joy J., George E., Vijayan P.P., Anas S., Thomas S. An overview of synthesis, morphology, and versatile applications of nanostructured graphitic carbon nitride (g-C3N4) J. Ind. Eng. Chem. 2024;133:74–89. doi: 10.1016/j.jiec.2023.12.016. - DOI
-
- Hayat A., Sohail M., Hamdy M.S., Taha T.A., AlSalem H.S., Alenad A.M., Amin M.A., Shah R., Palamanit A., Khan J., et al. Fabrication, characteristics, and applications of boron nitride and their composite nanomaterials. Surf. Interfaces. 2022;29:101725. doi: 10.1016/j.surfin.2022.101725. - DOI
-
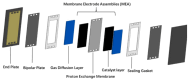
- Xing L., Shi W., Su H., Xu Q., Das P.K., Mao B., Scott K. Membrane electrode assemblies for PEM fuel cells: A review of functional graded design and optimization. Energy. 2019;177:445–464. doi: 10.1016/j.energy.2019.04.084. - DOI
-
- Shi D., Cai L., Zhang C., Chen D., Pan Z., Kang Z., Liu Y., Zhang J. Fabrication methods, structure design and durability analysis of advanced sealing materials in proton exchange membrane fuel cells. Chem. Eng. J. 2023;454:139995. doi: 10.1016/j.cej.2022.139995. - DOI
-
- Abdelkareem M.A., Wilberforce T., Elsaid K., Sayed E.T., Abdelghani E.A.M., Olabi A.G. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs) Int. J. Hydrogen Energy. 2021;46:23529–23547. doi: 10.1016/j.ijhydene.2020.08.250. - DOI
Publication types
LinkOut - more resources
Full Text Sources

