Synthesis and Antifungal Activity of 1,2,4-Oxadiazole Derivatives
- PMID: 40333860
- PMCID: PMC12029309
- DOI: 10.3390/molecules30081851
Synthesis and Antifungal Activity of 1,2,4-Oxadiazole Derivatives
Abstract
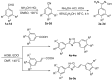
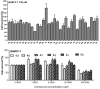
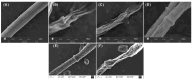
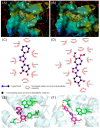
1,2,4-Oxadiazole derivatives containing anisic acid or cinnamic acid were designed and synthesized, which were expected to be an effective Succinate dehydrogenase (SDH) inhibitor, and their structures were characterized by 1H NMR, 13C NMR, and ESI-MS. The antifungal activity of the compounds against plant pathogenic fungi was screened by the mycelial growth inhibition test in vitro. Compounds 4f and 4q showed significant antifungal activities against Rhizoctonia solani (R. solani), Fusarium graminearum (F. graminearum), Exserohilum turcicum (E. turcicum), Botrytis cinerea (B. cinerea), and Colletotrichum capsica (C. capsica). The EC50 values of 4q were 38.88 μg/mL, 149.26 μg/mL, 228.99 μg/mL, and 41.67 μg/mL against R. solani, F. graminearum, E. turcicum, and C. capsica, respectively, and the EC50 values of 4f were 12.68 μg/mL, 29.97 μg/mL, 29.14 μg/mL, and 8.81 μg/mL, respectively. Compound 4f was better than commercial carbendazim against Exserohilum turcicum. Compounds 4f and 4q showed an antifungal effect on C. capsica of capsicum in vivo. Molecular docking simulation showed that 4f and 4q interacted with the target protein through the hydrogen bond and hydrophobic interaction, in which 4q can form hydrogen bonds with TRP173 and ILE27 of SDH, and 4f had hydrogen bonds with TYR58, TRP173, and SER39. This also explains the possible mechanism of action between the inhibitor and target protein.
Keywords: 1,2,4-oxadiazole derivatives; antifungal activity; biological evaluation; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Design, Synthesis, and Antifungal Evaluation of Diverse Heterocyclic Hydrazide Derivatives as Potential Succinate Dehydrogenase Inhibitors.J Agric Food Chem. 2024 Jun 12;72(23):12915-12924. doi: 10.1021/acs.jafc.3c08927. Epub 2024 May 28. J Agric Food Chem. 2024. PMID: 38807027
-
Design, Synthesis, and Antifungal Activities of Novel Pyrazole-4-carboxamide Derivatives Containing an Ether Group as Potential Succinate Dehydrogenase Inhibitors.J Agric Food Chem. 2023 Jun 21;71(24):9255-9265. doi: 10.1021/acs.jafc.3c00116. Epub 2023 Jun 7. J Agric Food Chem. 2023. PMID: 37283465
-
Rational design and discovery of novel hydrazide derivatives as potent succinate dehydrogenase inhibitors inspired by natural d/l-camphor.Pest Manag Sci. 2025 Feb;81(2):786-797. doi: 10.1002/ps.8481. Epub 2024 Oct 18. Pest Manag Sci. 2025. PMID: 39424965
-
Design, Synthesis, Antifungal Evaluation, and Three-Dimensional Quantitative Structure-Activity Relationship of Novel 5-Sulfonyl-1,3,4-thiadiazole Flavonoids.J Agric Food Chem. 2024 Oct 2;72(39):21419-21428. doi: 10.1021/acs.jafc.4c03505. Epub 2024 Sep 17. J Agric Food Chem. 2024. PMID: 39288935
-
Novel carboxylated pyrroline-2-one derivatives bearing a phenylhydrazine moiety: Design, synthesis, antifungal evaluation and 3D-QSAR analysis.Bioorg Med Chem Lett. 2020 Nov 1;30(21):127519. doi: 10.1016/j.bmcl.2020.127519. Epub 2020 Aug 27. Bioorg Med Chem Lett. 2020. PMID: 32860979
References
-
- United Nations 2019 Revision of World Population Prospects. [(accessed on 10 April 2025)]. Available online: https://population.un.org/wpp/
-
- Giovanni B., Roman P., Riccardo P., Loredana C., Giuseppe S., Dennis F., Stefania S., Stefano D., Angelo C., Filippo M. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018;122:308–315.
-
- Smith J., Waterhouse S., Paveley N. Evidence For Reduced Sexual Reproduction of Zymoseptoria Tritici Following Treatment with Fluxapyroxad and Implications for Initial Infection of Wheat Crops. Commun. Agric. Appl. Biol. Sci. 2014;79:385–395. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 2021ZDLSF03-05/the Key Research and Development Program of Shaanxi
- 23JC060/The Special Project for Serving the Local Area of the Shaanxi Provincial Department of Education
- 2022-85/the Youth Innovation Team Construction Project of Shaanxi Universities
- 2021TD07, 2021TD03/the Scientific and Technological Innovation Team project of Xi'an Medical University
- 23JP155/he Scientific Research Program Funded by Shaanxi Provincial Education Department
LinkOut - more resources
Full Text Sources

