Broussonetia papyrifera ameliorates imiquimod-induced psoriasis-like skin inflammation in mice by modulating the TLR4/NF-κB and PI3K/AKT signaling pathways
- PMID: 40333872
- PMCID: PMC12057870
- DOI: 10.1371/journal.pone.0322710
Broussonetia papyrifera ameliorates imiquimod-induced psoriasis-like skin inflammation in mice by modulating the TLR4/NF-κB and PI3K/AKT signaling pathways
Abstract
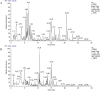
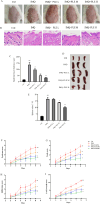
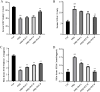
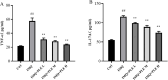
Psoriasis is a chronic, immune-mediated inflammatory skin disease, and the inflammatory response plays an important role in its development and progression. Psoriasis can appear at any age and occurs around the world. The pathogenesis of psoriasis has not been fully elucidated, and there is currently no effective treatment method in clinical practice. Broussonetia papyrifera is a traditional Chinese medicine that exhibited a significant therapeutic effect on psoriasis in our previous study due to its remarkable anti-inflammatory and anti-oxidant properties. However, its mechanism of action in treating psoriasis is still unclear. The purpose of this study is to evaluate the anti-psoriasis effect of the B. papyrifera leaves extract (PLE) in vivo and to explore its potential effects. PLE effectively alleviated imiquimod (IMQ)-induced psoriasis-like lesions, reduced psoriasis lesion area and severity index, decreased epidermal hyperplasia, ameliorated the oxidative stress-induced changes in the levels of superoxide dismutase (SOD) and malondialdehyde (MDA), and reduced the levels of the inflammatory cytokines TNF-α and IL-17A. PLE can also reduce the protein expression levels of TLR4, MyD88, p-NF-κBp65, p-IκBα, p-PI3K and p-AKT induced by IMQ model. Our findings suggest that PLE is effective in improving psoriasis-like symptoms, which might be ascribed to the inhibition of the TLR4/NF-κB and PI3K/AKT inflammation pathway. Our study demonstrates the potential mechanism of a natural source of PLE for the treatment of psoriasis. However, it is important to note that these findings lack clinical validation, and further studies are required to validate these results in clinical settings. Additionally, PLE shows potential in being a cost-effective alternative compared to existing biologics, which could have broader implications for psoriasis treatment in the future.
Copyright: © 2025 Huang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

