Zanubrutinib is well tolerated and effective in patients with CLL/SLL intolerant of ibrutinib/acalabrutinib: updated results
- PMID: 40334067
- PMCID: PMC12359222
- DOI: 10.1182/bloodadvances.2024015493
Zanubrutinib is well tolerated and effective in patients with CLL/SLL intolerant of ibrutinib/acalabrutinib: updated results
Abstract
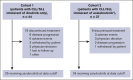
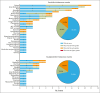
Bruton tyrosine kinase (BTK) inhibitors such as ibrutinib (ibr) revolutionized chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) treatment, although treatment-related toxicities limit the use of some BTK inhibitors. Zanubrutinib, a potent next-generation BTK inhibitor, has higher selectivity than ibrutinib or acalabrutinib. The ongoing phase 2, single-arm BGB-3111-215 study investigates the safety and efficacy of zanubrutinib in patients with B-cell malignancies who are intolerant of ibrutinib and/or acalabrutinib. Here, results in patients with CLL/SLL are presented. Patients received zanubrutinib 160 mg twice daily or 320 mg once a day. With a 34.5-month median follow-up, 71 patients (ibrutinib intolerant only, n = 44; acalabrutinib intolerant only, n = 17; ibrutinib and acalabrutinib intolerant, n = 10) received ≥1 zanubrutinib dose. On zanubrutinib, 54% (28/52) of ibrutinib-intolerant patients and 70% (19/27) of acalabrutinib-intolerant patients experienced no recurrence of intolerance adverse events (AEs); 60% and 72% of intolerance AEs did not recur, respectively. Of recurrent ibrutinib-intolerance AEs, 64% were lower grade; 44% of acalabrutinib-intolerance AEs were lower grade. No intolerance AEs recurred at a higher grade with zanubrutinib. The most common recurrent ibrutinib-intolerance and acalabrutinib-intolerance AEs were fatigue and diarrhea, respectively. The most common treatment-emergent AEs (TEAEs) with zanubrutinib were fatigue (32%) and COVID-19 (28%). Grade ≥3 TEAEs occurred in 61%, serious TEAEs in 32%, and TEAEs leading to discontinuation in 11%. Of 67 efficacy-evaluable patients, 94% experienced disease control: 30% had a best response of stable disease and 64% had a partial or complete response. These data demonstrate that patients intolerant of ibrutinib/acalabrutinib may benefit from switching to zanubrutinib therapy. This trial was registered at www.ClinicalTrials.gov as #NCT04116437.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.S. reports a consulting role and/or participation on advisory boards, steering committees, or data safety monitoring committees for AbbVie, Genentech, AstraZeneca, Genmab, Janssen, BeOne, Bristol Myers Squibb, MorphoSys/Incyte, Kite Pharma, Eli Lilly, Mustang Bio, Fate Therapeutics, Nurix, and Merck; received institutional research funding from Mustang Bio, Genentech, AbbVie, BeOne, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; reports stock options in Koi Biotherapeutics; and reports employment with Bristol Myers Squibb (spouse). J.M.B. received consultancy fees from AbbVie, Adaptive Biotechnologies, AstraZeneca, BeOne, Bristol Myers Squibb, Constellation, Eli Lilly, Epizyme, Foresight, Genentech, Genmab, Kura, Kymera, MorphoSys, Novartis, Nurix, TG Therapeutics, and Verastem; reports speakers bureau participation with BeOne; and received research funding from MorphoSys. J.C. reports employment with Florida Cancer Specialists & Research Institute; is a current equity holder in publicly traded company, American Oncology Network; and received research funding from Incyte, Takeda, Bristol Myers Squibb, Celgene, Acerta, BeOne, TG Therapeutics, Merck, Daiichi Sankyo, Novartis, MacroGenics, Eli Lilly, and Genentech. H.A.Y. reports speakers bureau participation with AbbVie, Amgen, AstraZeneca, BeOne, GlaxoSmithKline, Janssen, Karyopharm, and Takeda. C.M.F. served on an advisory board for Genmab/AbbVie; and speakers bureau for BeOne, Genentech, Genmab/AbbVie, Gilead/Kite, Incyte/MorphoSys, and Seagen. A.C. is a consultant to and equity holder in BeOne. H.Y., A.I., and Q.A. are employed by BeOne Medicines Ltd and may hold company stock/stock options. I.W.F. reports research grants (all payments made to institution) from AbbVie, AstraZeneca, BeOne Medicines Ltd, Bristol Myers Squibb, Celgene, City of Hope National Medical Center, Epizyme, Fate Therapeutics, Genentech, Gilead Sciences, IGM Biosciences, InnoCare Pharma, Incyte, Janssen, Kite Pharma, Loxo, Marker Therapeutics, Merck, MorphoSys, Myeloid Therapeutics, Novartis, Nurix, Pfizer, Roche, Seagen, TG Therapeutics, Vincerx, and 2seventy bio; reports consultancy fees (all payments made to physician) from AbbVie, BeOne, Genentech, Genmab, Kite Pharma, and Vincerx; and reports a role on an advisory committee for Vincerx. J.P.S. reports a consulting or advisory role with AbbVie, AstraZeneca, BeOne, Bristol Myers Squibb, Genentech, Janssen, Eli Lilly, and Merck. The remaining authors declare no competing financial interests.
Figures


References
-
- Shadman M. Diagnosis and treatment of chronic lymphocytic leukemia: a review. JAMA. 2023;329(11):918–932. - PubMed
-
- Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–1705. - PubMed
-
- Imbruvica (ibrutinib). Package insert. Pharmacyclics LLC; 2019.
-
- Calquence (acalabrutinib). Package insert. AstraZeneca Pharmaceuticals LP; 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

