Outcomes of brexucabtagene autoleucel in patients with relapsed/refractory acute lymphoblastic leukemia with CNS involvement
- PMID: 40334068
- PMCID: PMC12359224
- DOI: 10.1182/bloodadvances.2024015779
Outcomes of brexucabtagene autoleucel in patients with relapsed/refractory acute lymphoblastic leukemia with CNS involvement
Abstract
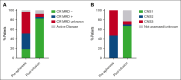
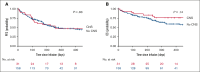
Patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) with central nervous system (CNS) involvement (CNS B-ALL) have poor outcomes and were frequently excluded from CD19-targeting chimeric antigen receptor (CAR) T-cell clinical trials. The efficacy and safety of brexucabtagene autoleucel (brexu-cel) in adults with R/R B-ALL was established by the ZUMA-3 trial, which excluded patients with advanced or symptomatic CNS involvement. In this retrospective multicenter analysis, we investigated the safety and efficacy of brexu-cel in patients with CNS B-ALL using data from the ROCCA (Real-World Outcomes Collaborative for CAR T in ALL) consortium. Of 189 patients who received infusion, 31 had CNS-2 (presence of blasts in cerebrospinal fluid with <5 white blood cells [WBCs] per μL) or CNS-3 (presence of blasts with >5 WBCs per μL and/or clinical signs/symptoms) disease before apheresis and are the focus of this report. The median age was 46.5 years (range, 24-76), and 58.1% were male. Most (87.1%) received bridging therapy. After brexu-cel, 21 of 24 patients with CNS restaging (87.5%) achieved CNS-1. Additionally, 28 of 30 evaluable patients achieved marrow complete remission; 25 were measurable residual disease negative. No statistically significant differences were seen in progression-free survival or overall survival after brexu-cel among patients with or without CNS involvement. Similarly, grade 3/4 immune effector cell-associated neurotoxicity syndrome occurred similarly in patients with (35.5%) and without (30%) CNS disease. In conclusion, our data suggest that brexu-cel results in high response rates in patients with CNS B-ALL, with toxicity comparable with that in patients without CNS involvement.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: G.W.R. served on an advisory board for Kite and Autolus. R.F. reports research funding from Kite/Gilead and Novartis; advisory board member role with Kite/Gilead and Autolus; and consulting role with Sanofi. N.M. reports membership on an entity’s board of directors or advisory committees for Anthem Inc. M.B. received research funding from Novartis and Fate Therapeutics. M.M.S. reports speakers bureau role with Bristol Myers Squibb (BMS). P.S. received honoraria from Autolus Therapeutics and BMS; and reports speakers bureau role with BMS and Sanofi. C.J.L. reports consultancy with Fresenius Kabi, Sanofi, and Incyte Corp; received honoraria from Kite Pharma, BMS, Sanofi, and Kadmon; served as an advisory board member with Kite Pharma, Sanofi, and Incyte Corp; reports speakers bureau role with Kite Pharma; and received research funding from Incyte Corp. A.C.L. reports research funding from Amgen, Astellas, Autolus Therapeutics, Kadmon, Kite/Gilead, Pharmacyclics, and Talaris; and consultancy with AbbVie, Amgen, Actinium, BMS, Pfizer, Sanofi, and Takeda. S.B.T. reports speakers bureau role with BMS and Jazz Pharmaceuticals; and served on an advisory board for Autolus. J.T.L. reports consultancy with Adaptive Biotechnologies, Pfizer, Kite/Gilead, and Takeda; and membership on an entity’s board of directors or advisory committees with, and travel, accommodations, and expenses from, Adaptive Biotechnologies. C.H.O. received research funding from Electra, Novartis, Arog, Orca Bio, Jazz Pharmaceuticals, Pfizer, and Seagen. M.S. reports consultancy with Jazz Pharmaceuticals, Kite, and Autolus. J.P.S. reports consultancy with Kite/Gilead Autolus, and Genmab. D.K. reports consultancy with, and research funding from, BMS. R.D.C. received research funding from Servier, Incyte, Kite/Gilead, Amgen, Vanda Pharmaceuticals, Jazz Pharmaceuticals, Merck, and Pfizer; served on advisory committees of PeproMene Bio and Autolus; reports consultancy with, and honoraria from, Kite/Gilead, Amgen, Jazz Pharmaceuticals, and Pfizer; and reports employment (spouse) with, and stock ownership in, Seagen, within the last 24 months. B.D.S. reports research funding from Incyte, Jazz Pharmaceuticals, Kite/Gilead, and Servier; received honoraria from Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, and Gilead Sciences; reports current employment with Moffitt Cancer Center; served on a data and safety monitoring board of, and received travel and accommodations and expenses from, Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, and Kite/Gilead; reports membership on an entity’s board of directors or advisory committees with PeproMene Bio; and reports consultancy with Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, and PeproMene Bio. I.A. reports consultancy with Kite, Sobi, Jazz, Pfizer, Amgen, and Takeda; received honoraria from Amgen; served on an advisory board with Amgen, Pfizer, Jazz, Kite, Takeda, Syndax, Sobi, and Wugen; and received research support from AbbVie and MacroGenics. L.S.M. reports consultancy with Amgen, Pfizer, Kite, Astellas, and Autolus; received research funding from BMS, Adaptive, Kite, Autolus, Astellas, Orca Bio, and Jasper; received honoraria from Kite; and reports membership on an entity’s board of directors or advisory committees with Adaptive. L.C.H. reports consulting for, and membership on an entity’s board of directors or advisory committees of, March Biosciences; serves as a speaker for Kite/Gilead; and reports honoraria from Sanofi. The remaining authors declare no competing financial interests.
Figures
References
-
- Surapaneni UR, Cortes JE, Thomas D, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia. Cancer. 2002;94(3):773–779. - PubMed
-
- Reman O, Pigneux A, Huguet F, et al. GET-LALA group Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis and/or at first relapse: results from the GET-LALA group. Leuk Res. 2008;32(11):1741–1750. - PubMed
-
- Fielding AK, Richards SM, Chopra R, et al. Medical Research Council of the United Kingdom Adult ALL Working Party. Eastern Cooperative Oncology Group Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–950. - PubMed
-
- Aldoss I, Otoukesh S, Zhang J, et al. Extramedullary disease relapse and progression after blinatumomab therapy for treatment of acute lymphoblastic leukemia. Cancer. 2022;128(3):529–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

