Pivotal results of SELECT-MDS-1 phase 3 study of tamibarotene with azacitidine in newly diagnosed higher-risk MDS
- PMID: 40334070
- PMCID: PMC12359220
- DOI: 10.1182/bloodadvances.2025016229
Pivotal results of SELECT-MDS-1 phase 3 study of tamibarotene with azacitidine in newly diagnosed higher-risk MDS
Abstract
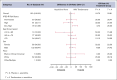
Higher-risk myelodysplastic syndrome (HR-MDS) with RARA gene overexpression is a subset of patients (pts) with an actionable target for tamibarotene, an oral and a selective retinoic acid receptor-α (RAR-α) agonist. Tamibarotene with azacitidine (AZA) showed complete remission (CR) rates in myeloid leukemia. SELECT-MDS-1 was a phase 3 study comparing the activity of tamibarotene + AZA to placebo + AZA in these pts with newly diagnosed HR-MDS with RARA overexpression. Eligible pts had confirmed RARA overexpression, untreated MDS with higher-risk features by revised International Prognostic Scoring System (IPSS-R), and marrow blast count >5%. Pts were randomized 2:1 to receive tamibarotene + AZA or placebo + AZA, respectively. A total of 246 participants were randomized with 164 and 82 in the tamibarotene + AZA and placebo + AZA groups, respectively. Baseline characteristics included: 69.9% male; median age 75 years (range, 38-93); primary MDS, 89.8%; MDS-excess blasts-1, 48% and MDS-excess blasts-2, 52%; and IPSS-R risk category intermediate (25.5%), high (35.7%), and very high (38.9%). The study did not meet the primary end point of CR, with a P value of .2084 for the treatment effect in the tamibarotene + AZA group. The CR rates were 23.81% and 18.75% in the tamibarotene + AZA and placebo + AZA groups, respectively. The use of tamibarotene-based therapy to target RAR-α as a novel approach in pts with HR-MDS with RARA gene overexpression is not a paradigm, which can augment response rates beyond AZA monotherapy. Further explorations of alternative approaches, including those with a biomarker, to alter the natural history of this disease are warranted. This trial was registered at www.clinicaltrials.gov as #NCT04797780.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.E.D. participated in advisory boards and/or had a consultancy with and received honoraria from Bristol Myers Squibb (BMS), Agios, and Novartis, and served on clinical trial committees or data safety monitoring boards for Novartis, Agios, AbbVie, Kura, Geron, Servier, Keros, Shattuck Labs, and BMS. S.T. participated in advisory boards or was a speaker for BMS, AbbVie, Astellas, and Novartis. A.P. participated in advisory board and/or received honoraria from Alexion, Pfizer, Novartis, Takeda, Grifols, Sobi, and Sanofi. D.D. received grants and/or research support from Alexion, Sobi, Amgen, Novartis, and Roche, and honoraria or fees for giving a talk from Daiichi-Sankyo, Incyte, Sobi, Novartis, Alexion, Amgen, and Roche. J.-M.T.-D. was a consultant on advisory boards for Novartis, SELLAS Life Sciences, BMS, Alexion, and Incyte; received travel funds from Alexion, Novartis, Sellas, and Pfizer; and received honoraria for consultancy from SELLAS Life Sciences. G.M. was a consultant, participated in advisory boards, or was a speaker for AbbVie, Astellas, AstraZeneca, Enable Life Sciences, Immunogen, Jazz, Janssen, Menarini/Stemline, Pfizer, Ryvu, Servier, Syros, Takeda, and UK Neqas, and received research support from AbbVie, Astellas, AstraZeneca, Daiichi Sankyo, and Pfizer. A.J. participated in advisory boards and/or reports consultancy with BMS, AbbVie, Novartis, and GlaxoSmithKline (GSK). A.M.Z. participated on advisory boards, consulted for and participated in clinical trial committees, and/or received honoraria from AbbVie, Akesobio, Agios, Amgen, Astellas, BioCryst, BeiGene, Boehringer-Ingelheim, Celgene/BMS, Chiesi, Daiichi Sankyo, Dr Reddy, Epizyme, Fibrogen, GSK, Glycomimetics, Genentech, Gilead, Geron, Janssen, Jasper, Karyopharm, Kyowa Kirin, Keros, Kura, Novartis, Notable, Orum, Otsuka, Pfizer, Regeneron, Rigel, Seattle Genetics, Schrodinger, Syros, Syndax, Servier, Takeda, Treadwell, Taiho, Vincerx, and Zentalis. S.D.-S. participated on advisory boards with Pfizer and reports travel grants with AbbVie. M.D.C. received honoraria/speaker fees from BMS, Novartis, Keros, and AbbVie, and participated on advisory boards with BMS, Agios, Hemavant, Syros, Keros, GSK, Curis, Astex/Otsuka, and Ascentage. W.C.-H. participated on advisory boards with BMS, Ascentage, and Replimune, and participated in clinical trials with AbbVie, Agios, Akesobio, BMS, Gilead, Incyte, Novartis, Syros, Shattuck Labs, and Sun Pharma. L.S. participated on advisory boards with BMS, AbbVie, Gilead, Novartis, Amgen, Pfizer, Takeda, Daiichi Sankyo, Servier, and Jazz. Y.O. reports consultancy with AbbVie and Medison, and participated on advisory boards with GSK, BMS, Astellas Gilead, and Novartis. P.K. received honoraria/conference support/advisory board fees from AbbVie, Astellas, Jazz, Gilead, Stemline-Menarini, Pfizer, Johnson & Johnson, and BMS-Celgene. M.L. participated on advisory boards for AbbVie, Astex Pharmaceuticals, Imago BioSciences, Janssen, Otsuka, and Syros, and received research support to institution from Janssen. G.B. reports speakers' bureau participation with, and travel support from, Novartis, Sanofi, AbbVie, Jazz, and Gilead. H.E.C. participated in clinical trial committees and/or received honoraria for consultancy with AbbVie, Agios, Amgen, BMS, Daiichi Sankyo, Geron, Kura, Jazz, Rigel, Novartis, Stemline, and Servier, and received research funding from Celgene. D.A.S. reports consultancy with AbbVie, Agios, Debiopharm, Janssen, Johnson & Johnson, Molecular Partners, and Novartis; served on advisory boards with AvenCell, Astellas, bluebird bio, BMS, Dark Blue Therapeutics, Geron, Shattuck Labs, Servier, Syndax, Syros, and Taiho Oncology; and received research funding from Aprea and Jazz. U.B. is a consultant for Novartis, AbbVie, Genentech, Incyte, Daiichi Sankyo, Sumitomo, Rigel, Astellas, Incyte, BMS, and Servier Scientific; participated on an advisory board with Vincerx Pharma; participated on a steering committee for BeiGene, Servier, and Sumitomo; and participated on a data monitoring committee for Takeda. V.S. participated on advisory boards for AbbVie, Ascentage, BMS, Geron, GSK, Keros, Jazz, Novartis, Otsuka, Syros, and Servier and received travel support from Janssen. J.M.Z. reports honoraria from Roche, Takeda, AbbVie, and Sobi, and received research support from BMS and Takeda. D.A.R., S.P., P.R., M.J.K., A.K., J.C., T.A.M., and V.M.K. are all employees of Syros Pharmaceuticals, Inc. T.C. reports consulting and board membership for Syros, AbbVie, BMS, and Novartis.
Figures
References
-
- Zeidan AM, Bewersdorf JP, Buckstein R, et al. Finding consistency in classifications of myeloid neoplasms: a perspective on behalf of the International Workshop for Myelodysplastic Syndromes. Leukemia. 2022;36(12):2939–2946. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous