Asciminib remained superior vs bosutinib in late-line CML-CP after nearly 4 years of follow-up in ASCEMBL
- PMID: 40334072
- PMCID: PMC12391778
- DOI: 10.1182/bloodadvances.2025016042
Asciminib remained superior vs bosutinib in late-line CML-CP after nearly 4 years of follow-up in ASCEMBL
Abstract
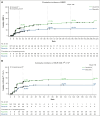
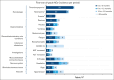
The efficacy of and disease control afforded by tyrosine kinase inhibitors (TKIs) in chronic myeloid leukemia in chronic phase (CML-CP) have led to increased longevity and thus the continued pursuit of alternative therapies that are efficacious and maximize tolerability. The 24- and 96-week analyses from ASCEMBL demonstrated superior efficacy, safety, and tolerability of asciminib when compared with bosutinib in later-line therapy, thereby meeting the primary and key secondary objectives. With nearly 4 years of follow-up, data from ASCEMBL continued to demonstrate the superior efficacy, safety, and tolerability of asciminib over bosutinib. At week 156, the major molecular response (MMR) rates remained higher with asciminib (33.8%) than with bosutinib (10.5%); the difference in MMR rates between arms, after adjusting for baseline major cytogenetic response, was 23.2% (95% confidence interval, 13.14-33.18; 2-sided P < .001). Asciminib continued to cause fewer grade ≥3 adverse events (AEs; 59.6% vs 68.4%) and fewer AEs that led to treatment discontinuation (8.3% vs 27.6%) than bosutinib. This updated analysis also includes patients who switched to asciminib because of a lack of efficacy with bosutinib. Two of the 25 patients who switched achieved MMR by the end of study, suggesting that earlier incorporation of asciminib, before other TKIs, may improve responses, albeit modestly. These long-term results further solidify asciminib as the therapy of choice for patients with CML-CP who were previously treated with ≥2 previous TKIs. This trial was registered at clinicaltrials.gov as #NCT03106779.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.H. received institutional research support from Novartis, Bristol Myers Squibb, Incyte, and Pfizer; and personal fees from Novartis and Incyte. D.R. received personal fees from Novartis, Pfizer, and Incyte. C.B. received grants from Novartis during the conduct of the study. Y.M. received honoraria from Bristol Myers Squibb, Novartis, Pfizer, and Astellas. J.E.C. received grants and consulting fees from Novartis and Pfizer; and grants from Bristol Myers Squibb. T.P.H. received grants and honoraria for serving on advisory boards and for symposia from Novartis and grants from Bristol Myers Squibb. J.F.A. received honoraria from Incyte and Paladin; received research funding from Incyte; received honoraria for lecturing from Novartis, Pfizer, and R-Pharm; and is an National Institute for Health and Care Research (NIHR) emeritus senior investigator and acknowledges the support of the Imperial NIHR Biomedical Research Centre. E.L. received grants from Novartis; and personal fees and nonfinancial support from Novartis, Pfizer, and Bristol Myers Squibb. S.V. received personal fees from Novartis, AbbVie, Janssen, Sanofi, BIOCAD, Takeda, and AstraZeneca; and nonfinancial support from Pfizer, Novartis, AbbVie, Janssen, Sanofi, and BIOCAD. D.-W.K. received grants from Novartis, Bristol Myers Squibb, Pfizer, ILYANG, and Takeda. A.A. received honoraria from Novartis and Takeda. P.l.C. received speaker’s honoraria from Novartis, Incyte, Pfizer, and Bristol Myers Squibb. K.S. received research funding and honoraria for serving on advisory boards from Novartis. D.D.H.K. received grants, honoraria, and consulting fees from Novartis; grants from Bristol Myers Squibb; and honoraria from Pfizer and Paladin. S.S. received honoraria, grants, and personal fees from Novartis; and grants and personal fees from Bristol Myers Squibb and Incyte. L.C. received honoraria for advisory boards from Otsuka and Novartis; consultancy fees from Keros Therapeutics; and honoraria from Otsuka, Novartis, and Keros Therapeutics. V.G.-G. received grants, nonfinancial support, and honoraria from Novartis, Pfizer, Bristol Myers Squibb, and Incyte. S.K., N.E., and V.D. report being employees of Novartis. M.J.M. reports receiving personal fees from Bristol Myers Squibb, Takeda, and Pfizer. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Asciminib in late-line CML treatment.Blood Adv. 2025 Aug 26;9(16):4317-4318. doi: 10.1182/bloodadvances.2025016874. Blood Adv. 2025. PMID: 40827887 Free PMC article. No abstract available.
References
-
- Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. - PubMed
-
- Druker BJ, Guilhot F, O'Brien SG, et al. IRIS Investigators Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

