Outcomes of Radium-223 and Stereotactic Ablative Radiotherapy Versus Stereotactic Ablative Radiotherapy for Oligometastatic Prostate Cancers: The RAVENS Phase II Randomized Trial
- PMID: 40334149
- PMCID: PMC12169860
- DOI: 10.1200/JCO-25-00131
Outcomes of Radium-223 and Stereotactic Ablative Radiotherapy Versus Stereotactic Ablative Radiotherapy for Oligometastatic Prostate Cancers: The RAVENS Phase II Randomized Trial
Abstract
Purpose: Randomized clinical trials (RCTs) have shown progression-free survival (PFS) benefits of metastasis-directed therapy (MDT) without androgen deprivation therapy for oligometastatic castration-sensitive prostate cancer (omCSPC). Most patients with bone metastatic (BM) omCSPC recur with additional bone disease after MDT. We hypothesized the BM-targeting alpha-emitter radium-223 dichloride (Ra223) could target subclinical bone disease and delay progression.
Methods: This is an investigator-initiated, multicenter, open-label phase II RCT. Eligible men with recurrent omCSPC with ≥one bone metastasis (≤three on conventional imaging and/or ≤five on molecular imaging) were randomly assigned (1:1) to stereotactic ablative radiation (SABR) MDT alone or SABR MDT with Ra223 (six cycles). Primary end point was composite PFS.
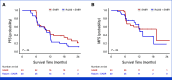
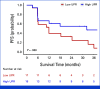
Results: From August 9, 2019, to March 2, 2023, 64 patients were randomly assigned, 33 to SABR MDT and 31 to SABR MDT/Ra223 balancing for key covariates. Most SABR MDT/Ra223 patients (87%) received six cycles of Ra223. The median PFS was 11.8 months with SABR MDT and 10.5 months with SABR MDT/Ra223 (adjusted hazard ratio [aHR], 1.42 [95% CI, 0.79 to 2.56]; P = .24). Seven patients (11%) experienced grade 3 treatment-related adverse events (no grade 4 or 5), 2 of 33 (6%) with SABR and 5 of 30 (17%) with SABR MDT/Ra223. Patients with high-risk (HiRi) pathogenic mutations in ATM, BRCA1/2, RB1, or TP53 had worse PFS (HR, 5.95 [95% CI, 1.83 to 19.3]; P = .003). Greater T-cell receptor (TCR) unique productive rearrangements were prognostic for improved PFS independent of the treatment arm (aHR, 0.45 [95% CI, 0.21 to 0.96]; P = .04).
Conclusion: Adding Ra223 to SABR MDT in BM omCSPC does not delay progression of disease. We provide evidence for an HiRi mutational signature and TCR repertoire as prognostic biomarkers in omCSPC treated with SABR MDT, highlighting the importance of collecting biological correlates in RCTs for omCSPC.
Trial registration: ClinicalTrials.gov NCT04037358.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:10. suppl 6.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

