Incidence, Characteristics, and Outcomes of Macular Neovascularization in Extensive Macular Atrophy with Pseudodrusen-like Appearance
- PMID: 40334199
- PMCID: PMC12366734
- DOI: 10.1097/IAE.0000000000004506
Incidence, Characteristics, and Outcomes of Macular Neovascularization in Extensive Macular Atrophy with Pseudodrusen-like Appearance
Abstract
Purpose: To report the incidence, features, and clinical outcomes of macular neovascularization (MNV) in a large Italian cohort of patients with extensive macular atrophy with pseudodrusen-like appearance (EMAP).
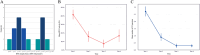
Methods: Retrospective, longitudinal study including 79 EMAP patients (158 eyes) with ≥6 months of follow-up at three retina clinics. Medical records and imaging were reviewed for demographic and clinical data, including age, best-corrected visual acuity (BCVA), MNV features, and retinal pigment epithelium (RPE) atrophy size, measured by short-wavelength autofluorescence and refined with near-infrared and OCT imaging. Main outcomes included cumulative MNV incidence, MNV risk factors, and BCVA and RPE atrophy changes in eyes with and without MNV.
Results: Over a mean follow-up of 40.4 months, MNV developed in 14 eyes (10 patients), with a 4-year cumulative incidence of 15.2%. Most MNVs were type 2 (86%) and subfoveal (64%). Cox regression identified younger age, fellow eye involvement, smaller RPE atrophy size, and greater central subfield thickness (all p<0.01) as significant risk factors for MNV. While eyes with MNV had lower baseline BCVA (58.4 vs. 71.4 letters, approximately 20/63 vs. 20/40 Snellen; p=0.005), BCVA decline over time was similar between the two groups (-3.9 vs. -4.1 letters/year, p=0.69). However, RPE atrophy progressed faster in MNV eyes (3.4 vs. 2.8 mm 2 /year, p=0.02).
Conclusions: In this EMAP cohort, MNV had a cumulative incidence of 15.2% at 4 years. Although BCVA outcomes were comparable, MNV was associated with faster atrophy progression, potentially due to a more aggressive disease phenotype or fibro-atrophic changes.
Keywords: CNV; EMAP; MNV; extensive macular atrophy with pseudodrusen-like appearance; incidence; macular neovascularization; outcomes; risk factors.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Opthalmic Communications Society, Inc.
Conflict of interest statement
Conflict of Interest: no conflicting relationship relevant to this research.
Figures




References
-
- Hamel CP, Meunier I, Arndt C, et al. Extensive macular atrophy with pseudodrusen-like appearance: a new clinical entity. Am J Ophthalmol 2009;147:609–620. - PubMed
-
- Douillard A, Picot MC, Delcourt C, et al. Clinical characteristics and risk factors of extensive macular atrophy with pseudodrusen: the EMAP case-control national clinical trial. Ophthalmology 2016;123:1865–1873. - PubMed
-
- Romano F, Cozzi M, Monteduro D, et al. Natural course and classification of extensive macular atrophy with pseudodrusen-like appearance. Retina 2023;43:402–411. - PubMed

