FTO facilitates colorectal cancer chemoresistance via regulation of NUPR1-dependent iron homeostasis
- PMID: 40334546
- PMCID: PMC12127581
- DOI: 10.1016/j.redox.2025.103647
FTO facilitates colorectal cancer chemoresistance via regulation of NUPR1-dependent iron homeostasis
Abstract
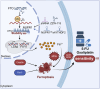
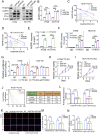
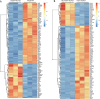
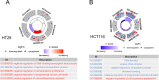
Drug resistance in colorectal cancer (CRC) poses a major challenge for cancer therapy and stands as the primary cause of cancer-related mortality. The N6-methyladenosine (m6A) modification has emerged as a pivotal regulator in cancer biology, yet the precise m6A regulators that propel CRC progression and chemoresistance remain elusive. Our study established a significant correlation between m6A regulatory gene expression profiles and CRC severity. Notably, based on the knockout cellular and mouse model created by CRISPR/Cas9-mediated genome engineering, we identified m6A demethylase FTO emerged as a pivotal orchestrator of CRC chemoresistance through the regulation of NUPR1, a critical transcription factor involved in iron homeostasis via LCN2 and FTH1. Mechanistic study revealed that FTO stabilized NUPR1 mRNA by specifically targeting the +451 m6A site, thereby preventing YTHDF2-mediated degradation of NUPR1 mRNA. Moreover, the simultaneous targeting of FTO and NUPR1 dramatically enhanced the efficacy of chemotherapy in CRC cells. Our findings underscore the potential of modulating the m6A methylome to overcome chemoresistance and highlight the FTO-NUPR1 axis as a critical determinant in CRC pathobiology.
Keywords: Chemoresistance; Colorectal cancer (CRC); Drug resistance; FTO (m6A demethylase); N6-methyladenosine (m6A) modification; NUPR1.
Copyright © 2025. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










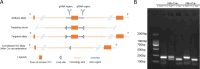

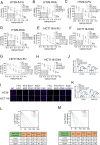


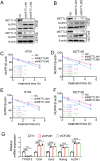


References
-
- Dekker E., et al. Colorectal cancer. Lancet (London, England) 2019;394:1467–1480. 10207. - PubMed
-
- Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics. CA Cancer J. Clin. 2024;74(1):12–49. 2024. - PubMed
-
- Pita-Fernández S., et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann. Oncol. : Official Journal of the European Society For Medical Oncology. 2015;26(4):644–656. - PubMed
-
- Johnston P.G., Kaye S. Capecitabine: a novel agent for the treatment of solid tumors. Anti Cancer Drugs. 2001;12(8):639–646. - PubMed
-
- Zaccara S., Ries R.J., Jaffrey S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019;20(10):608–624. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

