Neural and vascular contributions to sensory impairments in a human alpha-synuclein transgenic mouse model of Parkinson's disease
- PMID: 40334688
- PMCID: PMC12058711
- DOI: 10.1177/0271678X251338952
Neural and vascular contributions to sensory impairments in a human alpha-synuclein transgenic mouse model of Parkinson's disease
Abstract
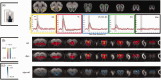
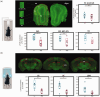
Parkinson's disease (PD) is a complex progressive neurodegenerative disorder involving hallmarks such as -Synuclein (Syn) aggregation and dopaminergic dysfunction that affect brain-wide neural activity. Although movement disorders are prominent in PD, sensory impairments also occur relatively early on, mainly in olfactory and, to a lesser extent visual systems. While these deficits have been described mainly at the behavioral and molecular levels, the underlying network-level activity remains poorly understood. Here, we harnessed a human Syn transgenic mouse model of PD with in vivo functional MRI (fMRI) to map evoked activity in the visual and olfactory pathways, along with pseudo-Continuous Arterial Spin Labeling (pCASL) and c-FOS measurements to disentangle vascular from neuronal effects. Upon stimulation with either odors or flickering lights, we found significant decreases in fMRI responses along both olfactory and visual pathways, in multiple cortical and subcortical sensory areas. Average Cerebral Blood Flow rates were decreased by ∼10% in the Syn group, while c-FOS levels were reduced by over 50%, suggesting a strong neural driver for the dysfunction, along with more modest vascular contributions. Our study provides insight into brain-level activity in an Syn-based model, and suggests a novel target for biomarking via quantification of simple sensory evoked responses.
Keywords: ASL; Parkinson’s disease; c-FOS; fMRI; α-Synuclein.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NS serves on the Scientific Advisory Board of Bruker Biospin.
Figures



Similar articles
-
Alterations in non-REM sleep and EEG spectra precede REM-sleep deficits in a model of synucleinopathy.J Parkinsons Dis. 2025 Mar;15(2):311-328. doi: 10.1177/1877718X241310723. Epub 2025 Jan 27. J Parkinsons Dis. 2025. PMID: 39973511
-
Reduced cerebral blood flow in an α-synuclein transgenic mouse model of Parkinson's disease.J Cereb Blood Flow Metab. 2020 Dec;40(12):2441-2453. doi: 10.1177/0271678X19895432. Epub 2019 Dec 19. J Cereb Blood Flow Metab. 2020. PMID: 31856640 Free PMC article.
-
Endothelial LRP1-ICD Accelerates Cognition-Associated Alpha-Synuclein Pathology and Neurodegeneration through PARP1 Activation in a Mouse Model of Parkinson's Disease.Mol Neurobiol. 2023 Feb;60(2):979-1003. doi: 10.1007/s12035-022-03119-4. Epub 2022 Nov 17. Mol Neurobiol. 2023. PMID: 36394710
-
Navigating the Neurobiology of Parkinson's: The Impact and Potential of α-Synuclein.Biomedicines. 2024 Sep 18;12(9):2121. doi: 10.3390/biomedicines12092121. Biomedicines. 2024. PMID: 39335634 Free PMC article. Review.
-
Alpha-Synuclein Contribution to Neuronal and Glial Damage in Parkinson's Disease.Int J Mol Sci. 2023 Dec 26;25(1):360. doi: 10.3390/ijms25010360. Int J Mol Sci. 2023. PMID: 38203531 Free PMC article. Review.
References
-
- Melzer TR, Watts R, MacAskill MR, et al.. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 2013; 80: 1841–1849. - PubMed
LinkOut - more resources
Full Text Sources

