Induction of Fructose Mediated De Novo Lipogenesis Coexists with the Upregulation of Mitochondrial Oxidative Function in Mice Livers
- PMID: 40334788
- PMCID: PMC12264563
- DOI: 10.1016/j.tjnut.2025.04.030
Induction of Fructose Mediated De Novo Lipogenesis Coexists with the Upregulation of Mitochondrial Oxidative Function in Mice Livers
Abstract
Background: Dysfunctional mitochondrial metabolism and sustained de novo lipogenesis (DNL) are characteristics of metabolic dysfunction-associated steatotic liver disease (MASLD), a comorbidity of obesity and type 2 diabetes. Fructose, a common sweetener and a potent inducer of lipogenesis, contributes to the etiology of MASLD.
Objectives: Our goal was to determine whether higher rates of DNL, through its biochemical relationships with mitochondria, can contribute to dysfunctional induction of oxidative networks in the liver.
Methods: Male C57BL/6JN mice were given a low-fat (10% fat kcal, 49.9% corn starch kcal), high-fat (HF; 60% fat kcal), or HF/high-fructose diet (HF/HFr; 25% fat kcal, 34.9% fructose kcal) for 24 wk. In a follow-up study, mice on normal feed pellets were provided either 30% fructose in drinking water (FW) to induce hepatic DNL or regular water (NW) for 14 d. Hepatic mitochondria and liver tissue were used to determine oxygen consumption, reactive oxygen species (ROS) generation, tricarboxylic acid (TCA) cycle activity, and gene/protein expression profiles.
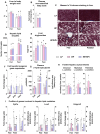
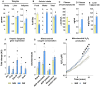
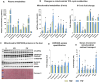
Results: Hepatic steatosis remained similar between HF and HF/HFr fed mice livers. However, lipogenic and lipid oxidation gene expression profiles and the induction of TCA cycle metabolism were all higher (P ≤ 0.05) in HF/HFr livers. Under fed conditions, the upregulation of DNL in FW livers occurred in concert with higher mitochondrial oxygen consumption (basal; 1.7 ± 0.21 compared with 3.3 ± 0.14 nmoles/min, P ≤ 0.05), higher ROS (0.87 ± 0.09 compared with 1.25 ± 0.12 μM, P ≤ 0.05) and higher flux through TCA cycle components P ≤0.05. Furthermore, TCA cycle activity and lipid oxidation remained higher during fasting in the FW livers P ≤ 0.05.
Conclusions: Our results show that fructose administration to mice led to the concurrent induction of mitochondrial oxidative networks and DNL in the liver. Sustained induction of both DNL and mitochondrial oxidative function could accelerate cellular stress and metabolic dysfunction during MASLD.
Keywords: de novo lipogenesis; fructose; ketogenesis; lipid oxidation; liver; mitochondrial metabolism; tricarboxylic acid cycle.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors report no conflicts of interest.
Figures




Similar articles
-
Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD.FASEB J. 2020 Nov;34(11):14832-14849. doi: 10.1096/fj.202001495R. Epub 2020 Sep 12. FASEB J. 2020. PMID: 32918763 Free PMC article.
-
Elevation of hepatic de novo lipogenesis in mice with overnutrition is dependent on multiple substrates.J Lipid Res. 2025 Jul;66(7):100838. doi: 10.1016/j.jlr.2025.100838. Epub 2025 Jun 9. J Lipid Res. 2025. PMID: 40499904 Free PMC article.
-
Gut microbiota mediates SREBP-1c-driven hepatic lipogenesis and steatosis in response to zero-fat high-sucrose diet.Mol Metab. 2025 Jul;97:102162. doi: 10.1016/j.molmet.2025.102162. Epub 2025 May 7. Mol Metab. 2025. PMID: 40345386 Free PMC article.
-
Dietary fructose: from uric acid to a metabolic switch in pediatric metabolic dysfunction-associated steatotic liver disease.Crit Rev Food Sci Nutr. 2025;65(23):4583-4598. doi: 10.1080/10408398.2024.2392150. Epub 2024 Aug 19. Crit Rev Food Sci Nutr. 2025. PMID: 39157959 Review.
-
Revisiting the concepts of de novo lipogenesis to understand the conversion of carbohydrates into fats: Stop overvaluing and extrapolating the renowned phrase "fat burns in the flame of carbohydrate".Nutrition. 2025 Feb;130:112617. doi: 10.1016/j.nut.2024.112617. Epub 2024 Oct 24. Nutrition. 2025. PMID: 39566326 Review.
References
-
- Patterson R.E., Kalavalapalli S., Williams C.M., Nautiyal M., Mathew J.T., Martinez J., et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am. J. Physiol. Endocrinol. Metab. 2016;310(7):E484–E494. doi: 10.1152/ajpendo.00492.2015. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

