Cellular crosstalk in fibrosis: Insights into macrophage and fibroblast dynamics
- PMID: 40334985
- PMCID: PMC12167814
- DOI: 10.1016/j.jbc.2025.110203
Cellular crosstalk in fibrosis: Insights into macrophage and fibroblast dynamics
Abstract
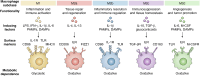
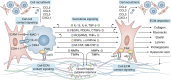
Pathological fibrosis, the excessive deposition of extracellular matrix and tissue stiffening that causes progressive organ dysfunction, underlies diverse chronic diseases. The fibrotic microenvironment is driven by the dynamic microenvironmental interaction between various cell types; macrophages and fibroblasts play central roles in fibrotic disease initiation, maintenance, and progression. Macrophage functional plasticity to microenvironmental stimuli modulates fibroblast functionality by releasing pro-inflammatory cytokines, growth factors, and matrix remodeling enzymes that promote fibroblast proliferation, activation, and differentiation into myofibroblasts. Activated fibroblasts and myofibroblasts serve as the fibrotic effector cells, secreting extracellular matrix components and initiating microenvironmental contracture. Fibroblasts also modulate macrophage function by releasing their own pro-inflammatory cytokines and growth factors, creating bidirectional crosstalk that reinforces the chronic fibrotic cycle. The intricate interplay between macrophages and fibroblasts, including their secretomes and signaling interactions, leads to tissue damage and pathological loss of tissue function. In this review, we examine macrophage-fibroblast reciprocal dynamic interactions in pathological fibrotic conditions. We discuss the specific lineages and functionality of macrophages and fibroblasts implicated in fibrotic progression, with focus on their signal transduction pathways and secretory signaling that enables their pro-fibrotic behavior. We then finish with a set of recommendations for future experimentation to develop a set of potential targets for anti-fibrotic therapeutic candidates. Understanding the cellular interactions between macrophages and fibroblasts provides valuable insights into potential therapeutic strategies to mitigate fibrotic disease progression.
Keywords: extracellular matrix; fibroblasts; fibrosis; inflammation; macrophages.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Bhattacharya M., Ramachandran P. Immunology of human fibrosis. Nat. Immunol. 2023;24:1423–1433. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

