Cyclooxygenase-2 negatively regulates osteogenic differentiation in murine bone marrow mesenchymal stem cells via the FOXO3a/p27kip1 pathway
- PMID: 40335058
- PMCID: PMC12058311
- DOI: 10.1302/2046-3758.145.BJR-2024-0262.R2
Cyclooxygenase-2 negatively regulates osteogenic differentiation in murine bone marrow mesenchymal stem cells via the FOXO3a/p27kip1 pathway
Abstract
Aims: Cyclooxygenase-2 (COX-2) is an enzyme that synthesizes prostaglandins from arachidonic acid. Previous reports have indicated that COX-2 is constitutively expressed in osteogenic cells instead of being expressed only after pathogenic induction, and that it facilitates osteoblast proliferation via PTEN/Akt/p27kip1 signalling. However, the role of COX-2 in osteogenic differentiation of murine bone marrow mesenchymal stromal cells (BMSCs) remains controversial. In this study, we investigated the function of COX-2 in the osteogenic differentiation of BMSCs.
Methods: COX-2 inhibitor, COX-2 overexpression vector, and p27kip1 small interfering RNA (siRNA) were used to evaluate the role of COX-2 in osteogenic differentiation and related signalling pathways in BMSCs.
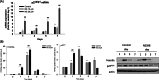
Results: We found that the messenger RNA (mRNA) and protein levels of COX-2 decreased gradually during osteogenic differentiation. Inhibition of COX-2 activity promoted FOXO3a and p27kip1 expression and simultaneously enhanced osteogenesis, as indicated by increased osteogenic gene expression and mineralization in BMSCs. Furthermore, when p27kip1 was silenced, the suppressive effects of COX-2 on osteogenesis were reversed. It demonstrated that the negative regulatory effect of COX-2 on osteogenesis was mediated by p27kip1. In addition, our results showed that overexpression of COX-2 reduced the mRNA and protein levels of FOXO3a and p27kip1, and thus attenuated osteogenic gene expression. These results indicate that COX-2 negatively regulates osteogenic differentiation by reducing the expression of osteogenic genes via the FOXO3a/p27kip1 signalling pathway.
Conclusion: Together with the findings from previous and current studies, these results indicate that COX-2 has a different role in proliferation versus differentiation during osteogenesis via FOXO3a/p27kip1 signalling in osteoblasts or BMSCs.
© 2025 Chuang et al.
Conflict of interest statement
All authors have read the journal’s policy on conflicts of interest and have no potential conflicts of interest to declare.
Figures






References
-
- Chang JK, Li CJ, Liao HJ, Wang CK, Wang GJ, Ho ML. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258(2–3):148–156. doi: 10.1016/j.tox.2009.01.016. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

