Biased antibodies and beyond: a new era in the diagnosis of PTH-dependent hypercalcemia
- PMID: 40335290
- PMCID: PMC12436076
- DOI: 10.1507/endocrj.EJ25-0051
Biased antibodies and beyond: a new era in the diagnosis of PTH-dependent hypercalcemia
Abstract
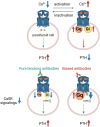
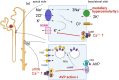
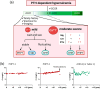
Hypercalcemia, a common electrolyte imbalance, requires accurate differential diagnosis to guide appropriate management. PTH-dependent hypercalcemia, predominantly caused by primary hyperparathyroidism (PHPT) and rarely by familial hypocalciuric hypercalcemia (FHH)-mainly due to heterozygous loss-of-function mutations in the CASR gene encoding the calcium-sensing receptor (CaSR)-now includes acquired hypocalciuric hypercalcemia (AHH) as an emerging disease entity. Initially identified as analogous to FHH, AHH was characterized by blocking antibodies targeting the CaSR. However, our research has identified unique autoantibodies, termed biased antibodies, that paradoxically regulate signaling by enhancing Gq activity while suppressing Gi activity. Investigating their mechanisms has not only provided insights into specific treatments for AHH but also suggested novel activation mechanisms and binding sites of the CaSR, offering a fresh perspective on the regulation of PTH secretion. In clinical practice, recognizing AHH is crucial. A key diagnostic feature is fluctuating serum calcium levels, making a wait-and-see approach viable for mild hypercalcemia. Conversely, hypercalcemic crises necessitate immediate diagnostic and therapeutic interventions. The most important diagnostic clue to differentiate AHH from PHPT is hypermagnesemia. Additionally, AHH is less likely to involve AVP resistance (i.e., nephrogenic diabetes insipidus) and acute kidney injury (AKI), owing to preserved medullary hyperosmolarity and minimal interference with AVP signaling. Finally, a relatively low PTH level serves as another distinguishing feature. Based on these observations, we propose a novel diagnostic guide for PTH-dependent hypercalcemia. We anticipate that this guide will help identify previously undiagnosed AHH cases in routine practice, enabling timely and effective management of this rare condition.
Keywords: Acquired hypocalciuric hypercalcemia (AHH); Biased antibodies; Calcimimetics; Calcium-sensing receptor (CaSR); PTH-dependent hypercalcemia.
Conflict of interest statement
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Noriko Makita is a member of
Figures




References
-
- Renaghan AD, Rosner MH (2018) Hypercalcemia: etiology and management. Nephrol Dial Transplant 33: 549–551.
-
- Walker MD, Shane E (2022) Hypercalcemia: A Review. JAMA 328: 1624–1636. - PubMed
-
- Potts JT, Jüppner H (2022) Disorders of the parathyroid gland and calcium homeostasis. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, et al. (eds) Harrison’s principles of internal medicine (21st) McGraw-Hill Education, New York, USA: 3169–3190.
-
- Motlaghzadeh Y, Bilezikian JP, Sellmeyer DE (2021) Rare causes of hypercalcemia: 2021 update. J Clin Endocrinol Metab 106: 3113–3128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

