Validation of the 5th edition of the World Health Organization and International Consensus Classification guidelines for TP53-mutated myeloid neoplasm in an independent international cohort
- PMID: 40335478
- PMCID: PMC12059121
- DOI: 10.1038/s41408-025-01290-0
Validation of the 5th edition of the World Health Organization and International Consensus Classification guidelines for TP53-mutated myeloid neoplasm in an independent international cohort
Abstract
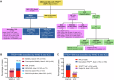
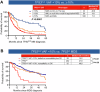
The World Health Organization (WHO-5) and International Consensus Classification (ICC) acknowledge the poor prognosis of TP53-mutated (TP53mut) myeloid neoplasm (MN). However, there are substantial differences between the two classifications that may lead to under- or overestimation of the prognostic risk. We retrospectively applied WHO-5 and ICC to 603 MN cases harboring TP53mut (variant allele frequency, VAF ≥ 2%). WHO-5 and ICC would not classify 64% and 20% of these cases as TP53mut MN, respectively. Moreover, of those classified, 67.5% would be classified discrepantly. Primary drivers of discrepancies included: (i) prognostic importance of TP53mut acute myeloid leukemia (AML), (ii) interaction of the blast percentage and allelic status, (iii) 17p.13.1 deletion detected by cytogenetics, (iv) complex karyotype (CK) as multi-hit equivalent, and (v) TP53mut VAF threshold, we analyzed survival outcomes of each of these groups with an aim to provide clarity. TP53mut AML was associated with significantly poor survival compared to TP53-wild type TP53wt AML, myelodysplasia-related (AML, MR 4.7 vs. 18.3 months; P < 0.0001), supporting its inclusion within TP53mut MN as a distinct subentity. Secondly, the survival of TP53mut with blast 10-19% was poor regardless of the allelic status. Thirdly, for cases with a single TP53mut with VAF < 50%, 17p13.1 del or CK serve as practical surrogates of biallelic inactivation, obviating the need for an additional copy number analysis. Finally, TP53mut AML, MDS multi-hit/multi-hit equivalent with VAF < 10% had significantly poorer survival compared to TP53mut MDS VAF < 10% without CK and 17p del, and were comparable to those with VAF ≥ 10% (14.1 vs. 48.8 vs.7.8 months, P < 0.0001). Collectively, these findings address key areas of contention and provide valuable insights that will guide future revisions of the WHO and ICC classifications.
© 2025. Crown.
Conflict of interest statement
Competing interests: MVS declares research funding to the institution from AbbVie, Astellas, Celgene, KURA Oncology, and Marker Therapeutics. D.H. is a member of the board of directors or advisory committees of AbbVie and Novartis. A.A.K. provides research support to Novartis and Astex. M.P. is a member of the board of directors or advisory committees of Stemline Therapeutics and receives research funding from Kura Oncology. P.G. is a member of the advisory board of AbbVie. N.G. has served on the Advisory Board for Agio and DISC Medicine. The remaining authors declare no competing interests.
Figures




Similar articles
-
TP53 mutation variant allele frequency of ≥10% is associated with poor prognosis in therapy-related myeloid neoplasms.Blood Cancer J. 2023 Apr 11;13(1):51. doi: 10.1038/s41408-023-00821-x. Blood Cancer J. 2023. PMID: 37041128 Free PMC article.
-
Evidence-based risk stratification of myeloid neoplasms harboring TP53 mutations.Blood Adv. 2025 Jul 8;9(13):3370-3380. doi: 10.1182/bloodadvances.2024015238. Blood Adv. 2025. PMID: 40085954 Free PMC article.
-
TP53 Mutation Status in Myelodysplastic Neoplasm and Acute Myeloid Leukemia: Impact of Reclassification Based on the 5th WHO and International Consensus Classification Criteria: A Korean Multicenter Study.Ann Lab Med. 2025 Mar 1;45(2):160-169. doi: 10.3343/alm.2024.0351. Epub 2024 Nov 5. Ann Lab Med. 2025. PMID: 39497415 Free PMC article.
-
TP53 -Mutated Myeloid Neoplasms: 2024 Update on Diagnosis, Risk-Stratification, and Management.Am J Hematol. 2025 Jun;100 Suppl 4(Suppl 4):88-115. doi: 10.1002/ajh.27655. Epub 2025 Mar 11. Am J Hematol. 2025. PMID: 40066944 Free PMC article. Review.
-
TP53 in MDS and AML: Biological and clinical advances.Cancer Lett. 2024 Apr 28;588:216767. doi: 10.1016/j.canlet.2024.216767. Epub 2024 Feb 27. Cancer Lett. 2024. PMID: 38417666 Review.
Cited by
-
Clinical and molecular characterization of TP53-mutant acute lymphoblastic leukemia in adults.Blood Cancer J. 2025 Aug 14;15(1):138. doi: 10.1038/s41408-025-01350-5. Blood Cancer J. 2025. PMID: 40813576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

