Dietary amino acids regulate Salmonella colonization via microbiota-dependent mechanisms in the mouse gut
- PMID: 40335509
- PMCID: PMC12058977
- DOI: 10.1038/s41467-025-59706-1
Dietary amino acids regulate Salmonella colonization via microbiota-dependent mechanisms in the mouse gut
Abstract
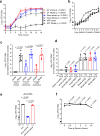
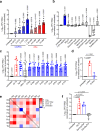
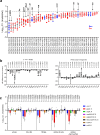
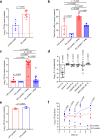
The gut microbiota confers host protection against pathogen colonization early after infection. Several mechanisms underlying the protection have been described, but the contributions of nutrient competition versus direct inhibition are controversial. Using an ex vivo model of Salmonella growth in the mouse cecum with its indigenous microbes, we find that nutrient limitation and typical inhibitory factors alone cannot prevent pathogen growth. However, the addition of certain amino acids markedly reverses the microbiota's ability to suppress pathogen growth. Enhanced Salmonella colonization after antibiotic treatment is ablated by exclusion of dietary protein, which requires the presence of the microbiota. Thus, dietary protein and amino acids are important regulators of colonization resistance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Salmonella virulence factors induce amino acid malabsorption in the ileum to promote ecosystem invasion of the large intestine.Proc Natl Acad Sci U S A. 2024 Nov 19;121(47):e2417232121. doi: 10.1073/pnas.2417232121. Epub 2024 Nov 15. Proc Natl Acad Sci U S A. 2024. PMID: 39546570 Free PMC article.
-
A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection.Cell Host Microbe. 2018 Aug 8;24(2):296-307.e7. doi: 10.1016/j.chom.2018.07.002. Epub 2018 Jul 26. Cell Host Microbe. 2018. PMID: 30057174 Free PMC article.
-
Antimicrobial peptide AP2 ameliorates Salmonella Typhimurium infection by modulating gut microbiota.BMC Microbiol. 2025 Feb 5;25(1):64. doi: 10.1186/s12866-025-03776-0. BMC Microbiol. 2025. PMID: 39910418 Free PMC article.
-
Salmonella versus the Microbiome.Microbiol Mol Biol Rev. 2020 Dec 23;85(1):e00027-19. doi: 10.1128/MMBR.00027-19. Print 2021 Feb 17. Microbiol Mol Biol Rev. 2020. PMID: 33361269 Free PMC article. Review.
-
Colonization resistance: the role of gut microbiota in preventing Salmonella invasion and infection.Gut Microbes. 2024 Jan-Dec;16(1):2424914. doi: 10.1080/19490976.2024.2424914. Epub 2024 Nov 8. Gut Microbes. 2024. PMID: 39514544 Free PMC article. Review.
References
-
- Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host-bacterial mutualism in the human intestine. Science307, 1915–1920 (2005). - PubMed

