Targeted metabolomic evaluation of peripheral blood mononucleated cells from patients with PMM2-CDG
- PMID: 40335571
- PMCID: PMC12059080
- DOI: 10.1038/s41598-025-98846-8
Targeted metabolomic evaluation of peripheral blood mononucleated cells from patients with PMM2-CDG
Abstract
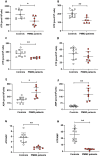
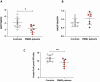
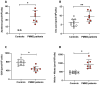
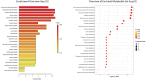
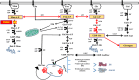
Phosphomannomutase-2 (PMM2) deficiency represents the most common congenital disorder of glycosylation (CDG). Currently, little is known about cell metabolic alterations occurring in these patients. Here, we quantified compounds connected to protein glycosylation (GDP-mannose, UDP-derivatives), energy metabolism (high-energy phosphates, nicotinic coenzymes, oxypurines), oxidative/nitrosative stress (GSH, nitrite, nitrate) and free amino acids in extracts of peripheral blood mononucleated cells (PBMCs), of seven PMM2-CDG patients and ten control healthy donors. Besides marked GDP-mannose decrease, PBMCs of PMM2-CDG patients had higher UDP-glucose (UDP-Glc), UDP-galactose (UDP-Gal) and UDP-Glucuronic levels, lower ATP, GTP and UTP levels, abnormal ATP/ADP, ATP/AMP and NAD+/NADH ratios, increased xanthine, uric acid and nitrite + nitrate levels, and decreased GSH and free amino acids concentrations. These results suggest a new, conceivable metabolic route leading to the increase of specific UDP-derivatives (UDP-Glc, UDP-Gal and UDP-Glucuronic), also potentially explaining the glycogen abnormalities recently found in PMM2-CDG patients. Altogether, this study highlighted various metabolic changes caused by PMM2 deficiency, illustrating the widespread effects of PMM2 mutations (beyond N-glycan biosynthesis) that may significantly vary depending on the cell line considered. Using PBMCs, as a cellular model of lower invasiveness than skin fibroblast, may advantage cell metabolism studies to investigate new therapies specifically targeted to PMM2 deficiency.
Keywords: Energy metabolism; HPLC; Peripheral blood mononucleated cells; Phosphomannomutase2 deficiency; Protein glycosylation; UDP-derivatives.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The study was conducted within a protocol approved by the CT1 Polyclinic Local Ethics Committee entitled: “Clinical evaluation in Phosphomannomutase deficiency” (approval number 221/2020), and written informed consent was obtained from all patients, according to the Declaration of Helsinki.
Figures


References
-
- Peanne, R. et al. Congenital disorders of glycosylation (CDG): quo vadis? Eur. J. Med. Genet.61, 643–663. 10.1016/j.ejmg.2017.10.012 (2018). - PubMed
-
- Ondruskova, N., Cechova, A., Hansikova, H., Honzik, T. & Jaeken, J. Congenital disorders of glycosylation: still hot in 2020. Biochim. Biophys. Acta Gen. Subj.1865, 129751. 10.1016/j.bbagen.2020.129751 (2021). - PubMed
-
- Mohorko, E., Glockshuber, R. & Aebi, M. Oligosaccharyltransferase: the central enzyme of N-linked protein glycosylation. J. Inherit. Metab. Dis.34, 869–878. 10.1007/s10545-011-9337-1 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

